Trung Q. Le
Multi-Scale Temporal Analysis for Failure Prediction in Energy Systems
Nov 05, 2024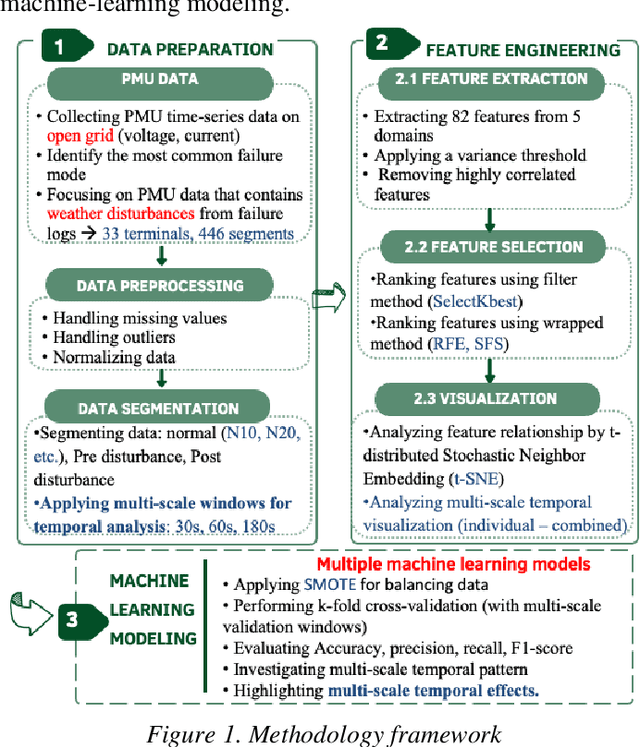
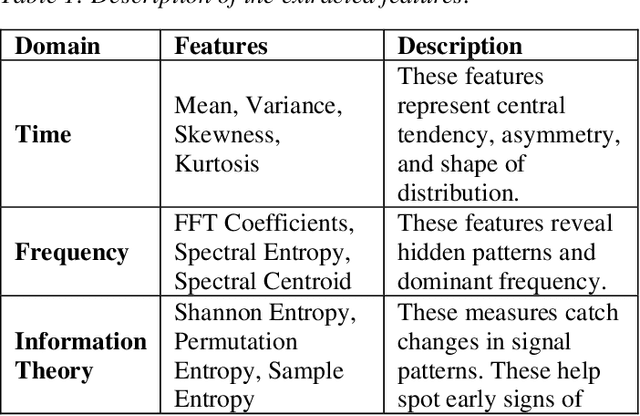
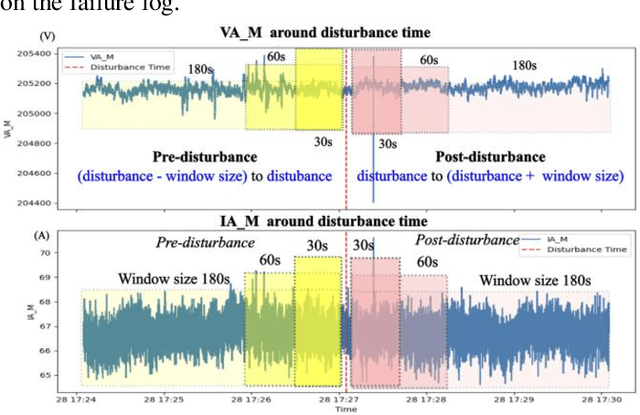
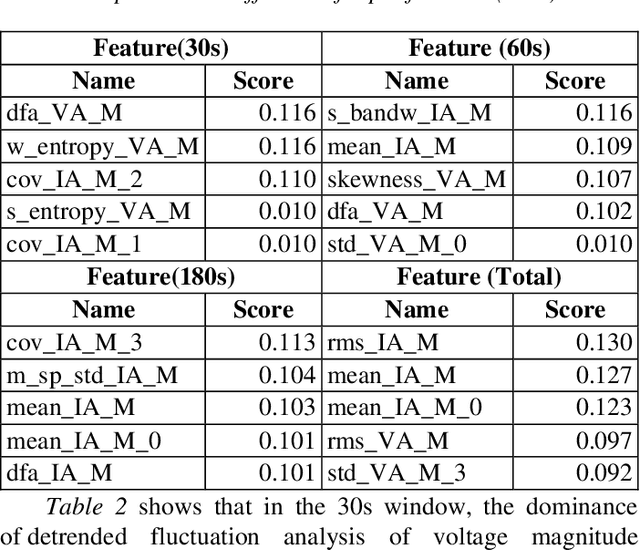
Abstract:Many existing models struggle to predict nonlinear behavior during extreme weather conditions. This study proposes a multi-scale temporal analysis for failure prediction in energy systems using PMU data. The model integrates multi-scale analysis with machine learning to capture both short-term and long-term behavior. PMU data lacks labeled states despite logged failure records, making it difficult to distinguish between normal and disturbance conditions. We address this through: (1) Extracting domain features from PMU time series data; (2) Applying multi-scale windows (30s, 60s, 180s) for pattern detection; (3) Using Recursive Feature Elimination to identify key features; (4) Training multiple machine learning models. Key contributions: Identifying significant features across multi-scale windows; Demonstrating LightGBM's superior performance (0.896 precision); Showing multi-scale analysis outperforms single-window models (0.841). Our work focuses on weather-related failures, with plans to extend to equipment failure and lightning events.
Multi-level Phenotypic Models of Cardiovascular Disease and Obstructive Sleep Apnea Comorbidities: A Longitudinal Wisconsin Sleep Cohort Study
Jun 19, 2024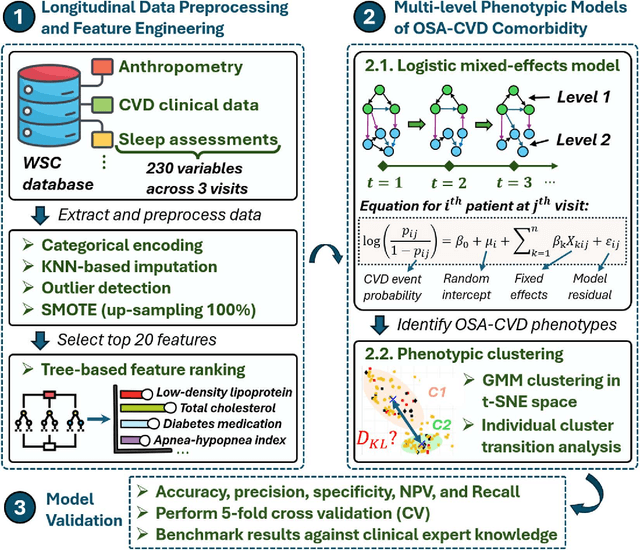
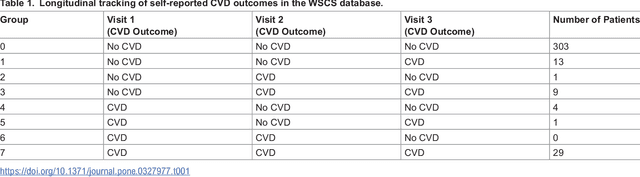
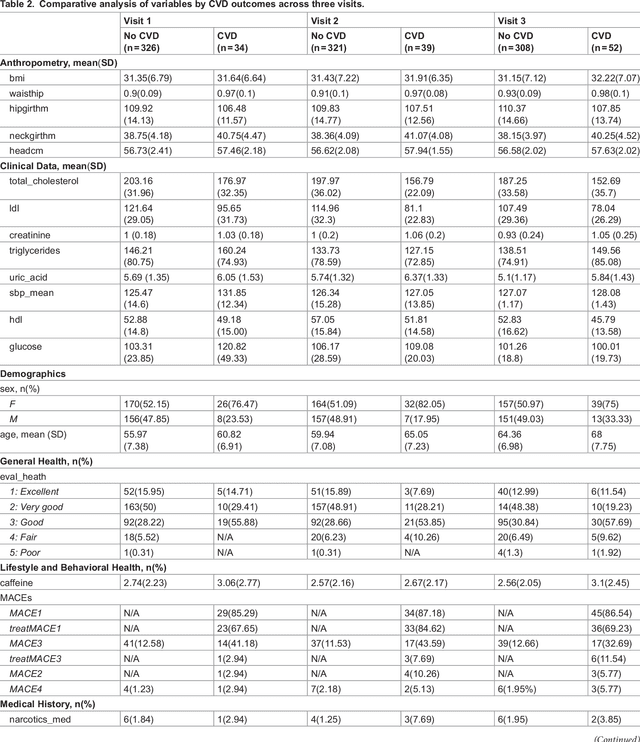
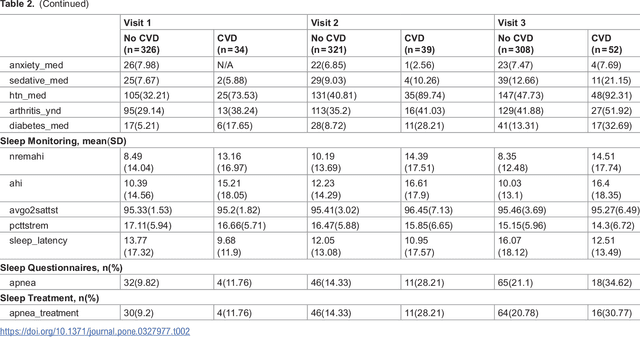
Abstract:Cardiovascular diseases (CVDs) are notably prevalent among patients with obstructive sleep apnea (OSA), posing unique challenges in predicting CVD progression due to the intricate interactions of comorbidities. Traditional models typically lack the necessary dynamic and longitudinal scope to accurately forecast CVD trajectories in OSA patients. This study introduces a novel multi-level phenotypic model to analyze the progression and interplay of these conditions over time, utilizing data from the Wisconsin Sleep Cohort, which includes 1,123 participants followed for decades. Our methodology comprises three advanced steps: (1) Conducting feature importance analysis through tree-based models to underscore critical predictive variables like total cholesterol, low-density lipoprotein (LDL), and diabetes. (2) Developing a logistic mixed-effects model (LGMM) to track longitudinal transitions and pinpoint significant factors, which displayed a diagnostic accuracy of 0.9556. (3) Implementing t-distributed Stochastic Neighbor Embedding (t-SNE) alongside Gaussian Mixture Models (GMM) to segment patient data into distinct phenotypic clusters that reflect varied risk profiles and disease progression pathways. This phenotypic clustering revealed two main groups, with one showing a markedly increased risk of major adverse cardiovascular events (MACEs), underscored by the significant predictive role of nocturnal hypoxia and sympathetic nervous system activity from sleep data. Analysis of transitions and trajectories with t-SNE and GMM highlighted different progression rates within the cohort, with one cluster progressing more slowly towards severe CVD states than the other. This study offers a comprehensive understanding of the dynamic relationship between CVD and OSA, providing valuable tools for predicting disease onset and tailoring treatment approaches.
Real-Time Magnetic Tracking and Diagnosis of COVID-19 via Machine Learning
Nov 01, 2023



Abstract:The COVID-19 pandemic underscored the importance of reliable, noninvasive diagnostic tools for robust public health interventions. In this work, we fused magnetic respiratory sensing technology (MRST) with machine learning (ML) to create a diagnostic platform for real-time tracking and diagnosis of COVID-19 and other respiratory diseases. The MRST precisely captures breathing patterns through three specific breath testing protocols: normal breath, holding breath, and deep breath. We collected breath data from both COVID-19 patients and healthy subjects in Vietnam using this platform, which then served to train and validate ML models. Our evaluation encompassed multiple ML algorithms, including support vector machines and deep learning models, assessing their ability to diagnose COVID-19. Our multi-model validation methodology ensures a thorough comparison and grants the adaptability to select the most optimal model, striking a balance between diagnostic precision with model interpretability. The findings highlight the exceptional potential of our diagnostic tool in pinpointing respiratory anomalies, achieving over 90% accuracy. This innovative sensor technology can be seamlessly integrated into healthcare settings for patient monitoring, marking a significant enhancement for the healthcare infrastructure.
A Probabilistic Domain-knowledge Framework for Nosocomial Infection Risk Estimation of Communicable Viral Diseases in Healthcare Personnel: A Case Study for COVID-19
Nov 05, 2021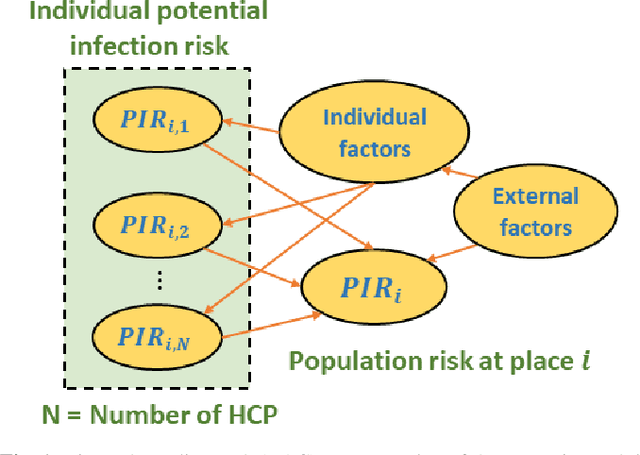
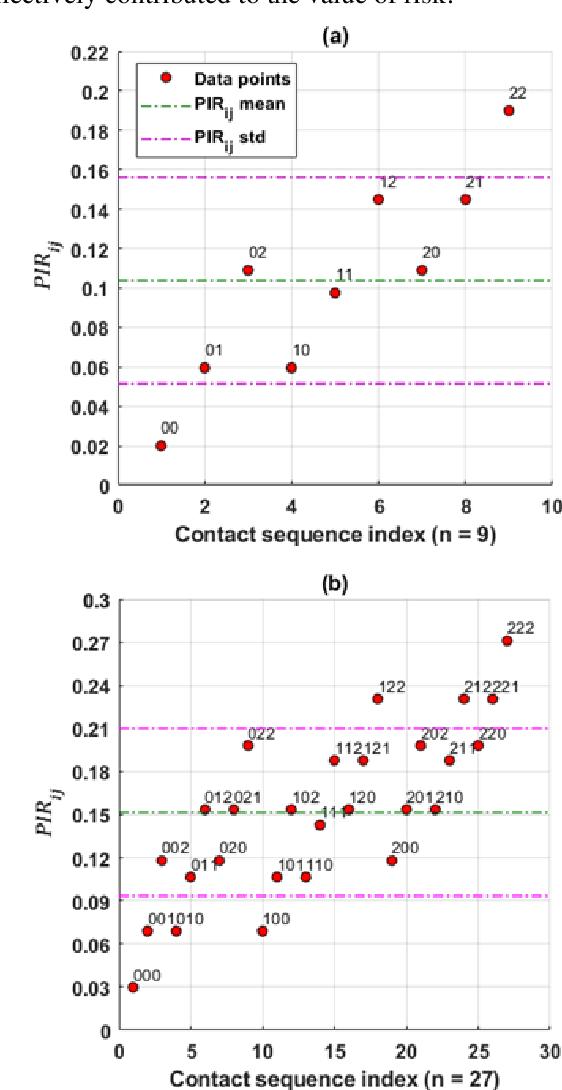
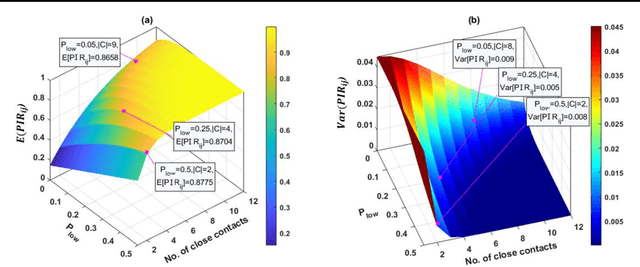

Abstract:Hospital-acquired infections of communicable viral diseases (CVDs) are posing a tremendous challenge to healthcare workers globally. Healthcare personnel (HCP) is facing a consistent risk of hospital-acquired infections, and subsequently higher rates of morbidity and mortality. We proposed a domain knowledge-driven infection risk model to quantify the individual HCP and the population-level healthcare facility risks. For individual-level risk estimation, a time-variant infection risk model is proposed to capture the transmission dynamics of CVDs. At the population-level, the infection risk is estimated using a Bayesian network model constructed from three feature sets including individual-level factors, engineering control factors, and administrative control factors. The sensitivity analyses indicated that the uncertainty in the individual infection risk can be attributed to two variables: the number of close contacts and the viral transmission probability. The model validation was implemented in the transmission probability model, individual level risk model, and population-level risk model using a Coronavirus disease case study. Regarding the first, multivariate logistic regression was applied for a cross-sectional data in the UK with an AIC value of 7317.70 and a 10-fold cross validation accuracy of 78.23%. For the second model, we collected laboratory-confirmed COVID-19 cases of HCP in different occupations. The occupation-specific risk evaluation suggested the highest-risk occupations were registered nurses, medical assistants, and respiratory therapists, with estimated risks of 0.0189, 0.0188, and 0.0176, respectively. To validate the population-level risk model, the infection risk in Texas and California was estimated. The proposed model will significantly influence the PPE allocation and safety plans for HCP
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge