Steve Y. Cho
PETAR: Localized Findings Generation with Mask-Aware Vision-Language Modeling for PET Automated Reporting
Oct 31, 2025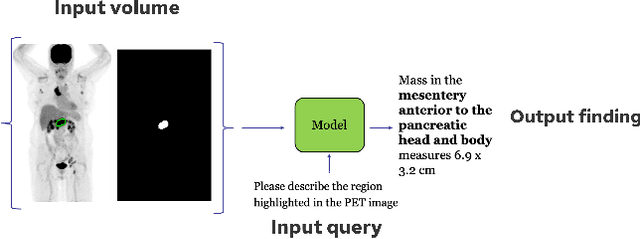
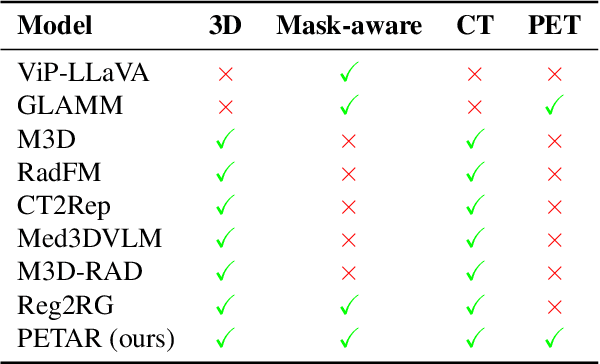

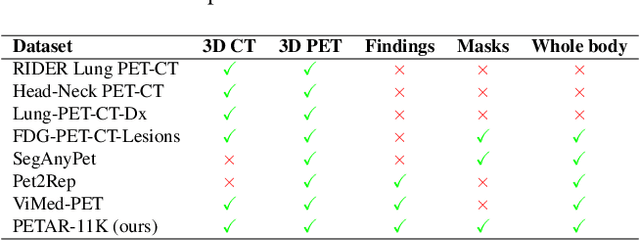
Abstract:Recent advances in vision-language models (VLMs) have enabled impressive multimodal reasoning, yet most medical applications remain limited to 2D imaging. In this work, we extend VLMs to 3D positron emission tomography and computed tomography (PET/CT), a domain characterized by large volumetric data, small and dispersed lesions, and lengthy radiology reports. We introduce a large-scale dataset comprising over 11,000 lesion-level descriptions paired with 3D segmentations from more than 5,000 PET/CT exams, extracted via a hybrid rule-based and large language model (LLM) pipeline. Building upon this dataset, we propose PETAR-4B, a 3D mask-aware vision-language model that integrates PET, CT, and lesion contours for spatially grounded report generation. PETAR bridges global contextual reasoning with fine-grained lesion awareness, producing clinically coherent and localized findings. Comprehensive automated and human evaluations demonstrate that PETAR substantially improves PET/CT report generation quality, advancing 3D medical vision-language understanding.
Automatic Quantification of Serial PET/CT Images for Pediatric Hodgkin Lymphoma Patients Using a Longitudinally-Aware Segmentation Network
Apr 12, 2024


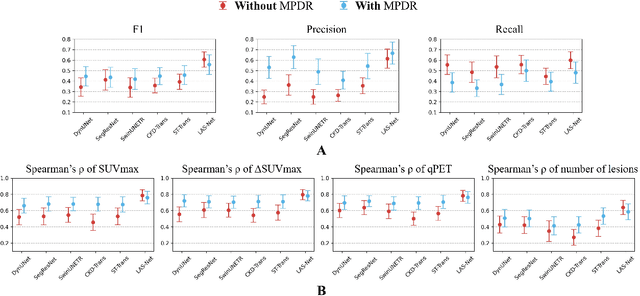
Abstract:$\textbf{Purpose}$: Automatic quantification of longitudinal changes in PET scans for lymphoma patients has proven challenging, as residual disease in interim-therapy scans is often subtle and difficult to detect. Our goal was to develop a longitudinally-aware segmentation network (LAS-Net) that can quantify serial PET/CT images for pediatric Hodgkin lymphoma patients. $\textbf{Materials and Methods}$: This retrospective study included baseline (PET1) and interim (PET2) PET/CT images from 297 patients enrolled in two Children's Oncology Group clinical trials (AHOD1331 and AHOD0831). LAS-Net incorporates longitudinal cross-attention, allowing relevant features from PET1 to inform the analysis of PET2. Model performance was evaluated using Dice coefficients for PET1 and detection F1 scores for PET2. Additionally, we extracted and compared quantitative PET metrics, including metabolic tumor volume (MTV) and total lesion glycolysis (TLG) in PET1, as well as qPET and $\Delta$SUVmax in PET2, against physician measurements. We quantified their agreement using Spearman's $\rho$ correlations and employed bootstrap resampling for statistical analysis. $\textbf{Results}$: LAS-Net detected residual lymphoma in PET2 with an F1 score of 0.606 (precision/recall: 0.615/0.600), outperforming all comparator methods (P<0.01). For baseline segmentation, LAS-Net achieved a mean Dice score of 0.772. In PET quantification, LAS-Net's measurements of qPET, $\Delta$SUVmax, MTV and TLG were strongly correlated with physician measurements, with Spearman's $\rho$ of 0.78, 0.80, 0.93 and 0.96, respectively. The performance remained high, with a slight decrease, in an external testing cohort. $\textbf{Conclusion}$: LAS-Net achieved high performance in quantifying PET metrics across serial scans, highlighting the value of longitudinal awareness in evaluating multi-time-point imaging datasets.
Automatic Personalized Impression Generation for PET Reports Using Large Language Models
Sep 18, 2023Abstract:Purpose: To determine if fine-tuned large language models (LLMs) can generate accurate, personalized impressions for whole-body PET reports. Materials and Methods: Twelve language models were trained on a corpus of PET reports using the teacher-forcing algorithm, with the report findings as input and the clinical impressions as reference. An extra input token encodes the reading physician's identity, allowing models to learn physician-specific reporting styles. Our corpus comprised 37,370 retrospective PET reports collected from our institution between 2010 and 2022. To identify the best LLM, 30 evaluation metrics were benchmarked against quality scores from two nuclear medicine (NM) physicians, with the most aligned metrics selecting the model for expert evaluation. In a subset of data, model-generated impressions and original clinical impressions were assessed by three NM physicians according to 6 quality dimensions and an overall utility score (5-point scale). Each physician reviewed 12 of their own reports and 12 reports from other physicians. Bootstrap resampling was used for statistical analysis. Results: Of all evaluation metrics, domain-adapted BARTScore and PEGASUSScore showed the highest Spearman's rho correlations (0.568 and 0.563) with physician preferences. Based on these metrics, the fine-tuned PEGASUS model was selected as the top LLM. When physicians reviewed PEGASUS-generated impressions in their own style, 89% were considered clinically acceptable, with a mean utility score of 4.08/5. Physicians rated these personalized impressions as comparable in overall utility to the impressions dictated by other physicians (4.03, P=0.41). Conclusion: Personalized impressions generated by PEGASUS were clinically useful, highlighting its potential to expedite PET reporting.
Domain-adapted large language models for classifying nuclear medicine reports
Mar 01, 2023



Abstract:With the growing use of transformer-based language models in medicine, it is unclear how well these models generalize to nuclear medicine which has domain-specific vocabulary and unique reporting styles. In this study, we evaluated the value of domain adaptation in nuclear medicine by adapting language models for the purpose of 5-point Deauville score prediction based on clinical 18F-fluorodeoxyglucose (FDG) PET/CT reports. We retrospectively retrieved 4542 text reports and 1664 images for FDG PET/CT lymphoma exams from 2008-2018 in our clinical imaging database. Deauville scores were removed from the reports and then the remaining text in the reports was used as the model input. Multiple general-purpose transformer language models were used to classify the reports into Deauville scores 1-5. We then adapted the models to the nuclear medicine domain using masked language modeling and assessed its impact on classification performance. The language models were compared against vision models, a multimodal vision language model, and a nuclear medicine physician with seven-fold Monte Carlo cross validation, reported are the mean and standard deviations. Domain adaption improved all language models. For example, BERT improved from 61.3% five-class accuracy to 65.7% following domain adaptation. The best performing model (domain-adapted RoBERTa) achieved a five-class accuracy of 77.4%, which was better than the physician's performance (66%), the best vision model's performance (48.1), and was similar to the multimodal model's performance (77.2). Domain adaptation improved the performance of large language models in interpreting nuclear medicine text reports.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge