Stefano Mandija
Quantitative mapping from conventional MRI using self-supervised physics-guided deep learning: applications to a large-scale, clinically heterogeneous dataset
Jan 08, 2026Abstract:Magnetic resonance imaging (MRI) is a cornerstone of clinical neuroimaging, yet conventional MRIs provide qualitative information heavily dependent on scanner hardware and acquisition settings. While quantitative MRI (qMRI) offers intrinsic tissue parameters, the requirement for specialized acquisition protocols and reconstruction algorithms restricts its availability and impedes large-scale biomarker research. This study presents a self-supervised physics-guided deep learning framework to infer quantitative T1, T2, and proton-density (PD) maps directly from widely available clinical conventional T1-weighted, T2-weighted, and FLAIR MRIs. The framework was trained and evaluated on a large-scale, clinically heterogeneous dataset comprising 4,121 scan sessions acquired at our institution over six years on four different 3 T MRI scanner systems, capturing real-world clinical variability. The framework integrates Bloch-based signal models directly into the training objective. Across more than 600 test sessions, the generated maps exhibited white matter and gray matter values consistent with literature ranges. Additionally, the generated maps showed invariance to scanner hardware and acquisition protocol groups, with inter-group coefficients of variation $\leq$ 1.1%. Subject-specific analyses demonstrated excellent voxel-wise reproducibility across scanner systems and sequence parameters, with Pearson $r$ and concordance correlation coefficients exceeding 0.82 for T1 and T2. Mean relative voxel-wise differences were low across all quantitative parameters, especially for T2 ($<$ 6%). These results indicate that the proposed framework can robustly transform diverse clinical conventional MRI data into quantitative maps, potentially paving the way for large-scale quantitative biomarker research.
Real-time myocardial landmark tracking for MRI-guided cardiac radio-ablation using Gaussian Processes
Jun 19, 2023Abstract:The high speed of cardiorespiratory motion introduces a unique challenge for cardiac stereotactic radio-ablation (STAR) treatments with the MR-linac. Such treatments require tracking myocardial landmarks with a maximum latency of 100 ms, which includes the acquisition of the required data. The aim of this study is to present a new method that allows to track myocardial landmarks from few readouts of MRI data, thereby achieving a latency sufficient for STAR treatments. We present a tracking framework that requires only few readouts of k-space data as input, which can be acquired at least an order of magnitude faster than MR-images. Combined with the real-time tracking speed of a probabilistic machine learning framework called Gaussian Processes, this allows to track myocardial landmarks with a sufficiently low latency for cardiac STAR guidance, including both the acquisition of required data, and the tracking inference. The framework is demonstrated in 2D on a motion phantom, and in vivo on volunteers and a ventricular tachycardia (arrhythmia) patient. Moreover, the feasibility of an extension to 3D was demonstrated by in silico 3D experiments with a digital motion phantom. The framework was compared with template matching - a reference, image-based, method - and linear regression methods. Results indicate an order of magnitude lower total latency (<10 ms) for the proposed framework in comparison with alternative methods. The root-mean-square-distances and mean end-point-distance with the reference tracking method was less than 0.8 mm for all experiments, showing excellent (sub-voxel) agreement. The high accuracy in combination with a total latency of less than 10 ms - including data acquisition and processing - make the proposed method a suitable candidate for tracking during STAR treatments.
Generalizable synthetic MRI with physics-informed convolutional networks
May 21, 2023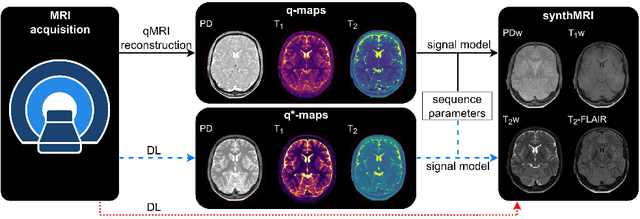

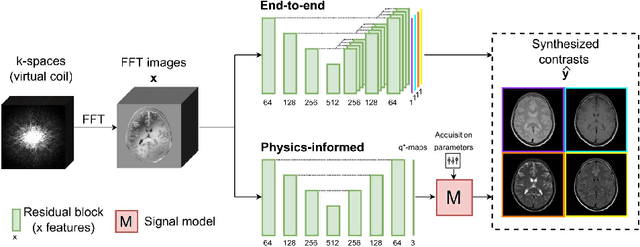

Abstract:In this study, we develop a physics-informed deep learning-based method to synthesize multiple brain magnetic resonance imaging (MRI) contrasts from a single five-minute acquisition and investigate its ability to generalize to arbitrary contrasts to accelerate neuroimaging protocols. A dataset of fifty-five subjects acquired with a standard MRI protocol and a five-minute transient-state sequence was used to develop a physics-informed deep learning-based method. The model, based on a generative adversarial network, maps data acquired from the five-minute scan to "effective" quantitative parameter maps, here named q*-maps, by using its generated PD, T1, and T2 values in a signal model to synthesize four standard contrasts (proton density-weighted, T1-weighted, T2-weighted, and T2-weighted fluid-attenuated inversion recovery), from which losses are computed. The q*-maps are compared to literature values and the synthetic contrasts are compared to an end-to-end deep learning-based method proposed by literature. The generalizability of the proposed method is investigated for five volunteers by synthesizing three non-standard contrasts unseen during training and comparing these to respective ground truth acquisitions via contrast-to-noise ratio and quantitative assessment. The physics-informed method was able to match the high-quality synthMRI of the end-to-end method for the four standard contrasts, with mean \pm standard deviation structural similarity metrics above 0.75 \pm 0.08 and peak signal-to-noise ratios above 22.4 \pm 1.9 and 22.6 \pm 2.1. Additionally, the physics-informed method provided retrospective contrast adjustment, with visually similar signal contrast and comparable contrast-to-noise ratios to the ground truth acquisitions for three sequences unused for model training, demonstrating its generalizability and potential application to accelerate neuroimaging protocols.
Acceleration Strategies for MR-STAT: Achieving High-Resolution Reconstructions on a Desktop PC within 3 minutes
May 04, 2022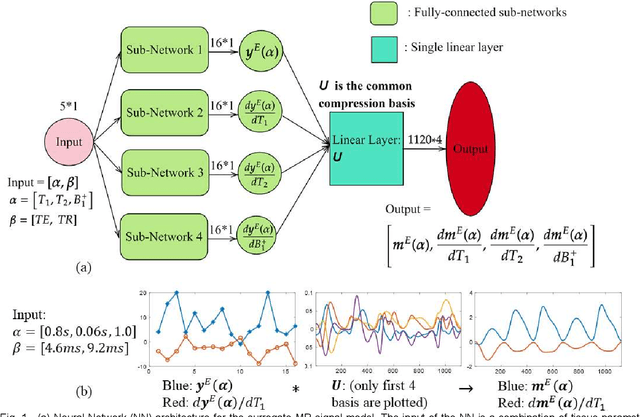
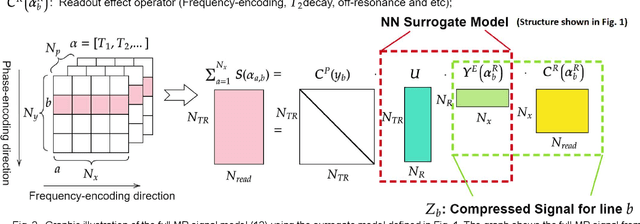
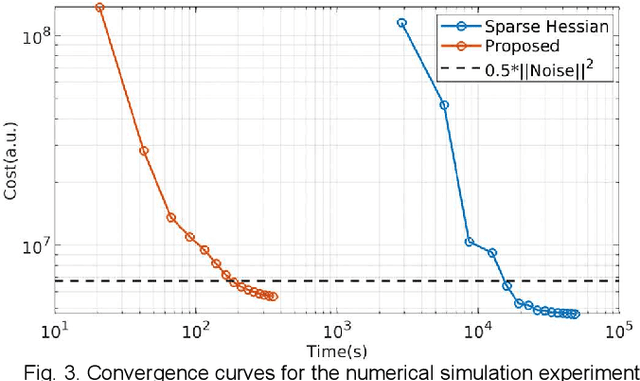
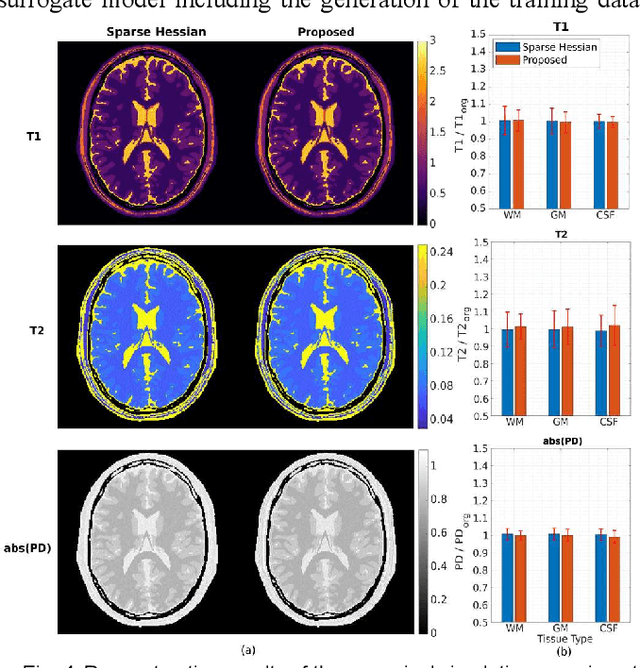
Abstract:MR-STAT is an emerging quantitative magnetic resonance imaging technique which aims at obtaining multi-parametric tissue parameter maps from single short scans. It describes the relationship between the spatial-domain tissue parameters and the time-domain measured signal by using a comprehensive, volumetric forward model. The MR-STAT reconstruction solves a large-scale nonlinear problem, thus is very computationally challenging. In previous work, MR-STAT reconstruction using Cartesian readout data was accelerated by approximating the Hessian matrix with sparse, banded blocks, and can be done on high performance CPU clusters with tens of minutes. In the current work, we propose an accelerated Cartesian MR-STAT algorithm incorporating two different strategies: firstly, a neural network is trained as a fast surrogate to learn the magnetization signal not only in the full time-domain but also in the compressed lowrank domain; secondly, based on the surrogate model, the Cartesian MR-STAT problem is re-formulated and split into smaller sub-problems by the alternating direction method of multipliers. The proposed method substantially reduces the computational requirements for runtime and memory. Simulated and in-vivo balanced MR-STAT experiments show similar reconstruction results using the proposed algorithm compared to the previous sparse Hessian method, and the reconstruction times are at least 40 times shorter. Incorporating sensitivity encoding and regularization terms is straightforward, and allows for better image quality with a negligible increase in reconstruction time. The proposed algorithm could reconstruct both balanced and gradient-spoiled in-vivo data within 3 minutes on a desktop PC, and could thereby facilitate the translation of MR-STAT in clinical settings.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge