Luuk Jacobs
FlowMRI-Net: A generalizable self-supervised physics-driven 4D Flow MRI reconstruction network for aortic and cerebrovascular applications
Oct 11, 2024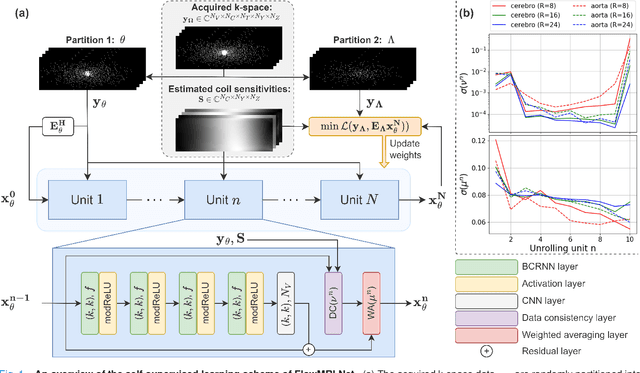
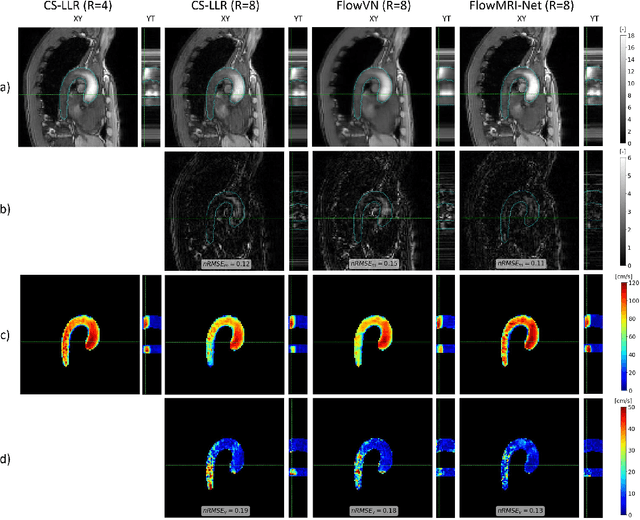
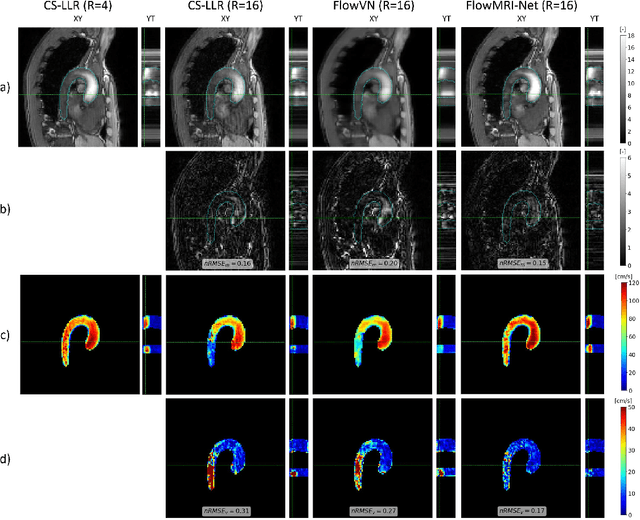
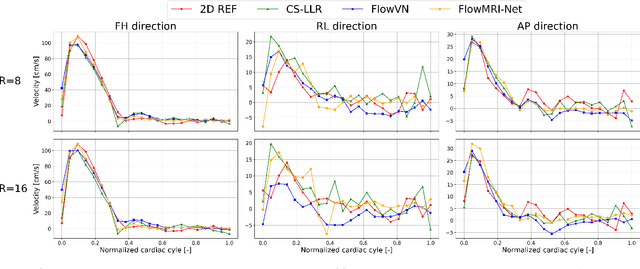
Abstract:In this work, we propose FlowMRI-Net, a novel deep learning-based framework for fast reconstruction of accelerated 4D flow magnetic resonance imaging (MRI) using physics-driven unrolled optimization and a complexvalued convolutional recurrent neural network trained in a self-supervised manner. The generalizability of the framework is evaluated using aortic and cerebrovascular 4D flow MRI acquisitions acquired on systems from two different vendors for various undersampling factors (R=8,16,24) and compared to state-of-the-art compressed sensing (CS-LLR) and deep learning-based (FlowVN) reconstructions. Evaluation includes quantitative analysis of image magnitudes, velocity magnitudes, and peak velocity curves. FlowMRINet outperforms CS-LLR and FlowVN for aortic 4D flow MRI reconstruction, resulting in vectorial normalized root mean square errors of $0.239\pm0.055$, $0.308\pm0.066$, and $0.302\pm0.085$ and mean directional errors of $0.023\pm0.015$, $0.036\pm0.018$, and $0.039\pm0.025$ for velocities in the thoracic aorta for R=16, respectively. Furthermore, FlowMRI-Net outperforms CS-LLR for cerebrovascular 4D flow MRI reconstruction, where no FlowVN can be trained due to the lack of a highquality reference, resulting in a consistent increase in SNR of around 6 dB and more accurate peak velocity curves for R=8,16,24. Reconstruction times ranged from 1 to 7 minutes on commodity CPU/GPU hardware. FlowMRI-Net enables fast and accurate quantification of aortic and cerebrovascular flow dynamics, with possible applications to other vascular territories. This will improve clinical adaptation of 4D flow MRI and hence may aid in the diagnosis and therapeutic management of cardiovascular diseases.
Generalizable synthetic MRI with physics-informed convolutional networks
May 21, 2023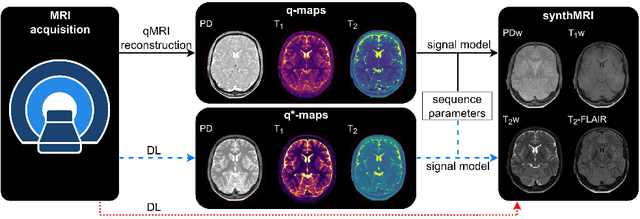

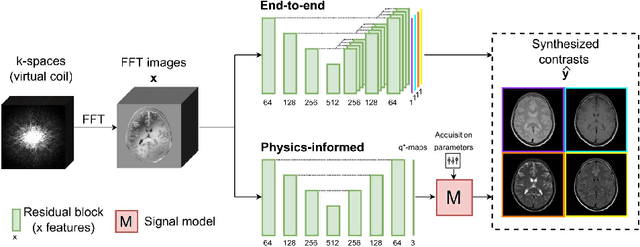

Abstract:In this study, we develop a physics-informed deep learning-based method to synthesize multiple brain magnetic resonance imaging (MRI) contrasts from a single five-minute acquisition and investigate its ability to generalize to arbitrary contrasts to accelerate neuroimaging protocols. A dataset of fifty-five subjects acquired with a standard MRI protocol and a five-minute transient-state sequence was used to develop a physics-informed deep learning-based method. The model, based on a generative adversarial network, maps data acquired from the five-minute scan to "effective" quantitative parameter maps, here named q*-maps, by using its generated PD, T1, and T2 values in a signal model to synthesize four standard contrasts (proton density-weighted, T1-weighted, T2-weighted, and T2-weighted fluid-attenuated inversion recovery), from which losses are computed. The q*-maps are compared to literature values and the synthetic contrasts are compared to an end-to-end deep learning-based method proposed by literature. The generalizability of the proposed method is investigated for five volunteers by synthesizing three non-standard contrasts unseen during training and comparing these to respective ground truth acquisitions via contrast-to-noise ratio and quantitative assessment. The physics-informed method was able to match the high-quality synthMRI of the end-to-end method for the four standard contrasts, with mean \pm standard deviation structural similarity metrics above 0.75 \pm 0.08 and peak signal-to-noise ratios above 22.4 \pm 1.9 and 22.6 \pm 2.1. Additionally, the physics-informed method provided retrospective contrast adjustment, with visually similar signal contrast and comparable contrast-to-noise ratios to the ground truth acquisitions for three sequences unused for model training, demonstrating its generalizability and potential application to accelerate neuroimaging protocols.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge