Shunpan Liang
Combating the Bucket Effect:Multi-Knowledge Alignment for Medication Recommendation
Apr 25, 2025Abstract:Medication recommendation is crucial in healthcare, offering effective treatments based on patient's electronic health records (EHR). Previous studies show that integrating more medication-related knowledge improves medication representation accuracy. However, not all medications encompass multiple types of knowledge data simultaneously. For instance, some medications provide only textual descriptions without structured data. This imbalance in data availability limits the performance of existing models, a challenge we term the "bucket effect" in medication recommendation. Our data analysis uncovers the severity of the "bucket effect" in medication recommendation. To fill this gap, we introduce a cross-modal medication encoder capable of seamlessly aligning data from different modalities and propose a medication recommendation framework to integrate Multiple types of Knowledge, named MKMed. Specifically, we first pre-train a cross-modal encoder with contrastive learning on five knowledge modalities, aligning them into a unified space. Then, we combine the multi-knowledge medication representations with patient records for recommendations. Extensive experiments on the MIMIC-III and MIMIC-IV datasets demonstrate that MKMed mitigates the "bucket effect" in data, and significantly outperforms state-of-the-art baselines in recommendation accuracy and safety.
Medication Recommendation via Dual Molecular Modalities and Multi-Substructure Distillation
May 30, 2024Abstract:Medication recommendation combines patient medical history with biomedical knowledge to assist doctors in determining medication combinations more accurately and safely. Existing approaches based on molecular knowledge overlook the atomic geometric structure of molecules, failing to capture the high-dimensional characteristics and intrinsic physical properties of medications, leading to structural confusion and the inability to extract useful substructures from individual patient visits. To address these limitations, we propose BiMoRec, which overcomes the inherent lack of molecular essential information in 2D molecular structures by incorporating 3D molecular structures and atomic properties. To retain the fast response required of recommendation systems, BiMoRec maximizes the mutual information between the two molecular modalities through bimodal graph contrastive learning, achieving the integration of 2D and 3D molecular graphs, and finally distills substructures through interaction with single patient visits. Specifically, we use deep learning networks to construct a pre-training method to obtain representations of 2D and 3D molecular structures and substructures, and we use contrastive learning to derive mutual information. Subsequently, we generate fused molecular representations through a trained GNN module, re-determining the relevance of substructure representations in conjunction with the patient's clinical history information. Finally, we generate the final medication combination based on the extracted substructure sequences. Our implementation on the MIMIC-III and MIMIC-IV datasets demonstrates that our method achieves state-of-the-art performance. Compared to the next best baseline, our model improves accuracy by 1.8\% while maintaining the same level of DDI as the baseline.
Knowledge-Aware Multi-Intent Contrastive Learning for Multi-Behavior Recommendation
Apr 18, 2024



Abstract:Multi-behavioral recommendation optimizes user experiences by providing users with more accurate choices based on their diverse behaviors, such as view, add to cart, and purchase. Current studies on multi-behavioral recommendation mainly explore the connections and differences between multi-behaviors from an implicit perspective. Specifically, they directly model those relations using black-box neural networks. In fact, users' interactions with items under different behaviors are driven by distinct intents. For instance, when users view products, they tend to pay greater attention to information such as ratings and brands. However, when it comes to the purchasing phase, users become more price-conscious. To tackle this challenge and data sparsity problem in the multi-behavioral recommendation, we propose a novel model: Knowledge-Aware Multi-Intent Contrastive Learning (KAMCL) model. This model uses relationships in the knowledge graph to construct intents, aiming to mine the connections between users' multi-behaviors from the perspective of intents to achieve more accurate recommendations. KAMCL is equipped with two contrastive learning schemes to alleviate the data scarcity problem and further enhance user representations. Extensive experiments on three real datasets demonstrate the superiority of our model.
Relationship Discovery for Drug Recommendation
Apr 18, 2024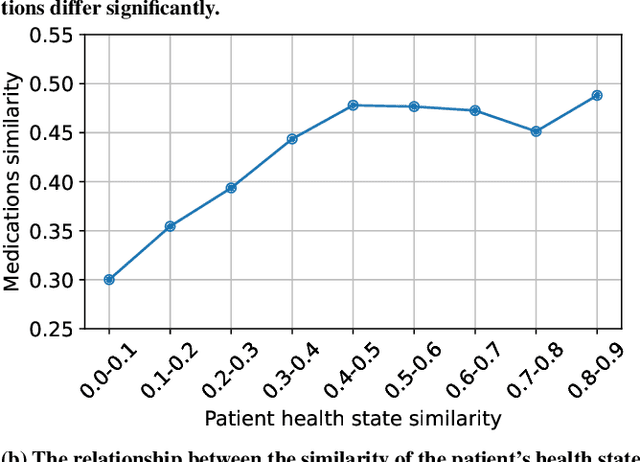
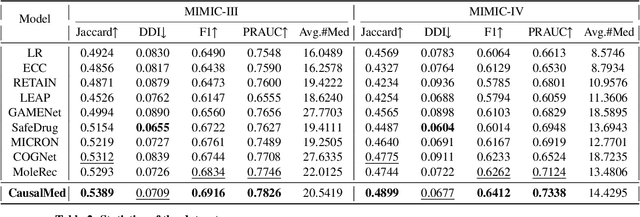
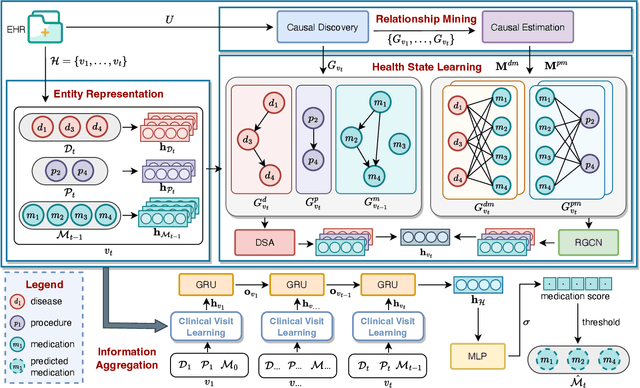
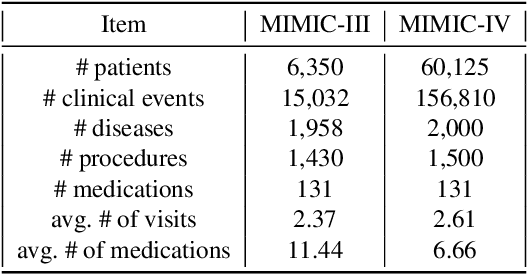
Abstract:Medication recommendation systems are designed to deliver personalized drug suggestions that are closely aligned with individual patient needs. Previous studies have primarily concentrated on developing medication embeddings, achieving significant progress. Nonetheless, these approaches often fall short in accurately reflecting individual patient profiles, mainly due to challenges in distinguishing between various patient conditions and the inability to establish precise correlations between specific conditions and appropriate medications. In response to these issues, we introduce DisMed, a model that focuses on patient conditions to enhance personalization. DisMed employs causal inference to discern clear, quantifiable causal links. It then examines patient conditions in depth, recognizing and adapting to the evolving nuances of these conditions, and mapping them directly to corresponding medications. Additionally, DisMed leverages data from multiple patient visits to propose combinations of medications. Comprehensive testing on real-world datasets demonstrates that DisMed not only improves the customization of patient profiles but also surpasses leading models in both precision and safety.
Dual-Granularity Medication Recommendation Based on Causal Inference
Mar 01, 2024



Abstract:As medical demands grow and machine learning technology advances, AI-based diagnostic and treatment systems are garnering increasing attention. Medication recommendation aims to integrate patients' long-term health records with medical knowledge, recommending accuracy and safe medication combinations for specific conditions. However, most existing researches treat medication recommendation systems merely as variants of traditional recommendation systems, overlooking the heterogeneity between medications and diseases. To address this challenge, we propose DGMed, a framework for medication recommendation. DGMed utilizes causal inference to uncover the connections among medical entities and presents an innovative feature alignment method to tackle heterogeneity issues. Specifically, this study first applies causal inference to analyze the quantified therapeutic effects of medications on specific diseases from historical records, uncovering potential links between medical entities. Subsequently, we integrate molecular-level knowledge, aligning the embeddings of medications and diseases within the molecular space to effectively tackle their heterogeneity. Ultimately, based on relationships at the entity level, we adaptively adjust the recommendation probabilities of medication and recommend medication combinations according to the patient's current health condition. Experimental results on a real-world dataset show that our method surpasses existing state-of-the-art baselines in four evaluation metrics, demonstrating superior performance in both accuracy and safety aspects. Compared to the sub-optimal model, our approach improved accuracy by 4.40%, reduced the risk of side effects by 6.14%, and increased time efficiency by 47.15%.
StratMed: Relevance Stratification for Low-resource Medication Recommendation
Sep 06, 2023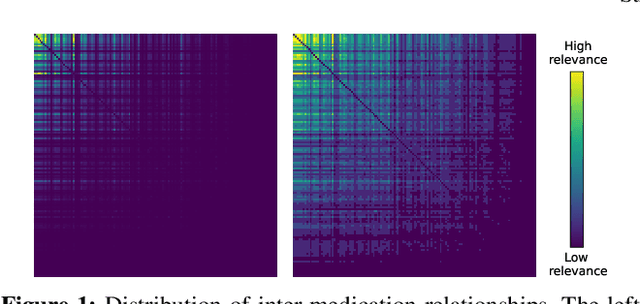
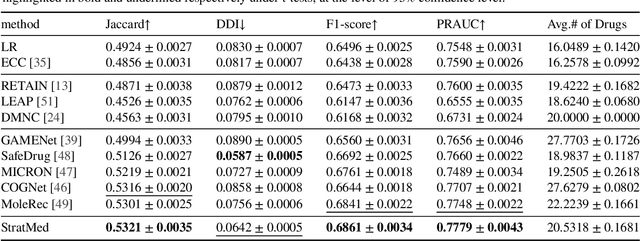
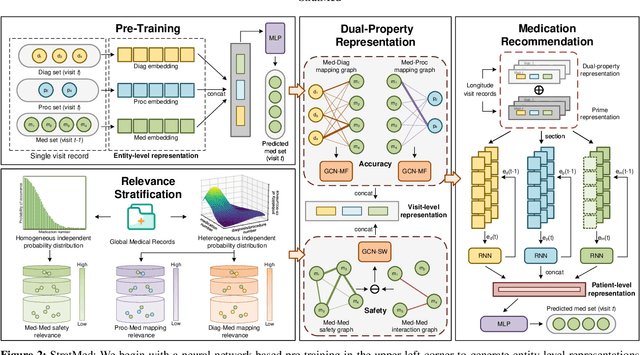
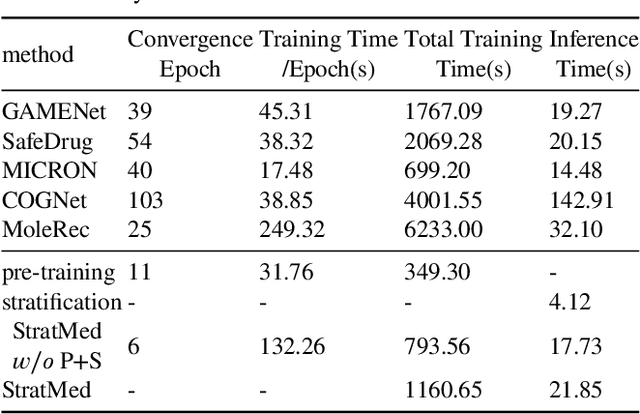
Abstract:With the growing imbalance between limited medical resources and escalating demands, AI-based clinical tasks have become paramount. Medication recommendation, as a sub-domain, aims to amalgamate longitudinal patient history with medical knowledge, assisting physicians in prescribing safer and more accurate medication combinations. Existing methods overlook the inherent long-tail distribution in medical data, lacking balanced representation between head and tail data, which leads to sub-optimal model performance. To address this challenge, we introduce StratMed, a model that incorporates an innovative relevance stratification mechanism. It harmonizes discrepancies in data long-tail distribution and strikes a balance between the safety and accuracy of medication combinations. Specifically, we first construct a pre-training method using deep learning networks to obtain entity representation. After that, we design a pyramid-like data stratification method to obtain more generalized entity relationships by reinforcing the features of unpopular entities. Based on this relationship, we designed two graph structures to express medication precision and safety at the same level to obtain visit representations. Finally, the patient's historical clinical information is fitted to generate medication combinations for the current health condition. Experiments on the MIMIC-III dataset demonstrate that our method has outperformed current state-of-the-art methods in four evaluation metrics (including safety and accuracy).
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge