Shuja Khalid
GaussianVAE: Adaptive Learning Dynamics of 3D Gaussians for High-Fidelity Super-Resolution
Jun 09, 2025Abstract:We present a novel approach for enhancing the resolution and geometric fidelity of 3D Gaussian Splatting (3DGS) beyond native training resolution. Current 3DGS methods are fundamentally limited by their input resolution, producing reconstructions that cannot extrapolate finer details than are present in the training views. Our work breaks this limitation through a lightweight generative model that predicts and refines additional 3D Gaussians where needed most. The key innovation is our Hessian-assisted sampling strategy, which intelligently identifies regions that are likely to benefit from densification, ensuring computational efficiency. Unlike computationally intensive GANs or diffusion approaches, our method operates in real-time (0.015s per inference on a single consumer-grade GPU), making it practical for interactive applications. Comprehensive experiments demonstrate significant improvements in both geometric accuracy and rendering quality compared to state-of-the-art methods, establishing a new paradigm for resolution-free 3D scene enhancement.
SurGNN: Explainable visual scene understanding and assessment of surgical skill using graph neural networks
Aug 24, 2023



Abstract:This paper explores how graph neural networks (GNNs) can be used to enhance visual scene understanding and surgical skill assessment. By using GNNs to analyze the complex visual data of surgical procedures represented as graph structures, relevant features can be extracted and surgical skill can be predicted. Additionally, GNNs provide interpretable results, revealing the specific actions, instruments, or anatomical structures that contribute to the predicted skill metrics. This can be highly beneficial for surgical educators and trainees, as it provides valuable insights into the factors that contribute to successful surgical performance and outcomes. SurGNN proposes two concurrent approaches -- one supervised and the other self-supervised. The paper also briefly discusses other automated surgical skill evaluation techniques and highlights the limitations of hand-crafted features in capturing the intricacies of surgical expertise. We use the proposed methods to achieve state-of-the-art results on EndoVis19, and custom datasets. The working implementation of the code can be found at https://github.com/<redacted>.
RefiNeRF: Modelling dynamic neural radiance fields with inconsistent or missing camera parameters
Mar 15, 2023Abstract:Novel view synthesis (NVS) is a challenging task in computer vision that involves synthesizing new views of a scene from a limited set of input images. Neural Radiance Fields (NeRF) have emerged as a powerful approach to address this problem, but they require accurate knowledge of camera \textit{intrinsic} and \textit{extrinsic} parameters. Traditionally, structure-from-motion (SfM) and multi-view stereo (MVS) approaches have been used to extract camera parameters, but these methods can be unreliable and may fail in certain cases. In this paper, we propose a novel technique that leverages unposed images from dynamic datasets, such as the NVIDIA dynamic scenes dataset, to learn camera parameters directly from data. Our approach is highly extensible and can be integrated into existing NeRF architectures with minimal modifications. We demonstrate the effectiveness of our method on a variety of static and dynamic scenes and show that it outperforms traditional SfM and MVS approaches. The code for our method is publicly available at \href{https://github.com/redacted/refinerf}{https://github.com/redacted/refinerf}. Our approach offers a promising new direction for improving the accuracy and robustness of NVS using NeRF, and we anticipate that it will be a valuable tool for a wide range of applications in computer vision and graphics.
wildNeRF: Complete view synthesis of in-the-wild dynamic scenes captured using sparse monocular data
Sep 20, 2022



Abstract:We present a novel neural radiance model that is trainable in a self-supervised manner for novel-view synthesis of dynamic unstructured scenes. Our end-to-end trainable algorithm learns highly complex, real-world static scenes within seconds and dynamic scenes with both rigid and non-rigid motion within minutes. By differentiating between static and motion-centric pixels, we create high-quality representations from a sparse set of images. We perform extensive qualitative and quantitative evaluation on existing benchmarks and set the state-of-the-art on performance measures on the challenging NVIDIA Dynamic Scenes Dataset. Additionally, we evaluate our model performance on challenging real-world datasets such as Cholec80 and SurgicalActions160.
Advances in Prediction of Readmission Rates Using Long Term Short Term Memory Networks on Healthcare Insurance Data
Jun 30, 2022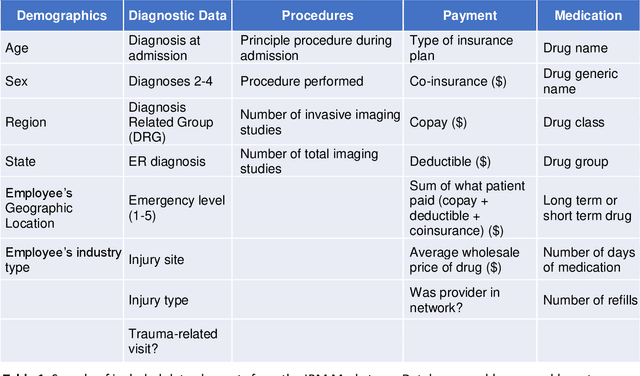
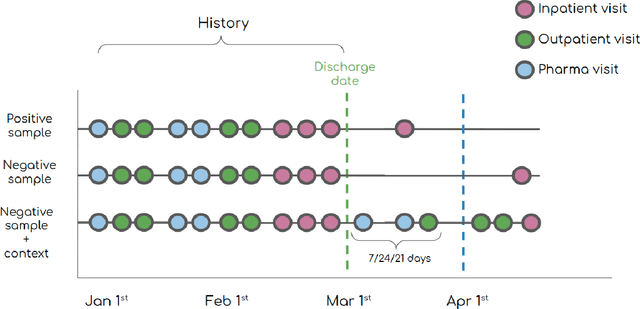
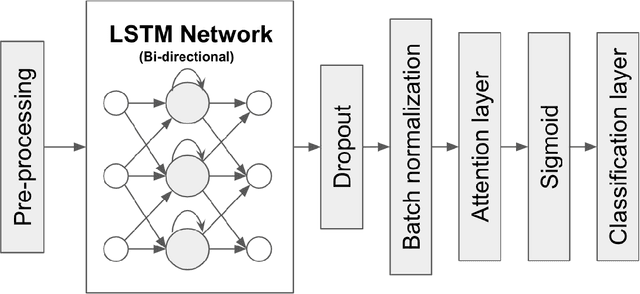
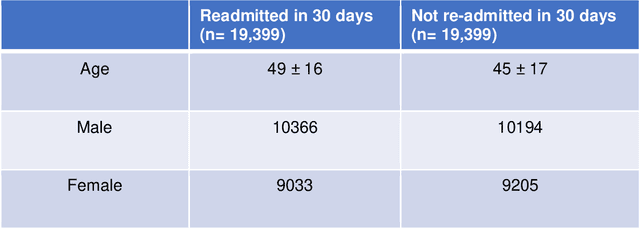
Abstract:30-day hospital readmission is a long standing medical problem that affects patients' morbidity and mortality and costs billions of dollars annually. Recently, machine learning models have been created to predict risk of inpatient readmission for patients with specific diseases, however no model exists to predict this risk across all patients. We developed a bi-directional Long Short Term Memory (LSTM) Network that is able to use readily available insurance data (inpatient visits, outpatient visits, and drug prescriptions) to predict 30 day re-admission for any admitted patient, regardless of reason. The top-performing model achieved an ROC AUC of 0.763 (0.011) when using historical, inpatient, and post-discharge data. The LSTM model significantly outperformed a baseline random forest classifier, indicating that understanding the sequence of events is important for model prediction. Incorporation of 30-days of historical data also significantly improved model performance compared to inpatient data alone, indicating that a patients clinical history prior to admission, including outpatient visits and pharmacy data is a strong contributor to readmission. Our results demonstrate that a machine learning model is able to predict risk of inpatient readmission with reasonable accuracy for all patients using structured insurance billing data. Because billing data or equivalent surrogates can be extracted from sites, such a model could be deployed to identify patients at risk for readmission before they are discharged, or to assign more robust follow up (closer follow up, home health, mailed medications) to at-risk patients after discharge.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge