Sabine Bergler
Comparing and combining some popular NER approaches on Biomedical tasks
May 30, 2023Abstract:We compare three simple and popular approaches for NER: 1) SEQ (sequence-labeling with a linear token classifier) 2) SeqCRF (sequence-labeling with Conditional Random Fields), and 3) SpanPred (span-prediction with boundary token embeddings). We compare the approaches on 4 biomedical NER tasks: GENIA, NCBI-Disease, LivingNER (Spanish), and SocialDisNER (Spanish). The SpanPred model demonstrates state-of-the-art performance on LivingNER and SocialDisNER, improving F1 by 1.3 and 0.6 F1 respectively. The SeqCRF model also demonstrates state-of-the-art performance on LivingNER and SocialDisNER, improving F1 by 0.2 F1 and 0.7 respectively. The SEQ model is competitive with the state-of-the-art on the LivingNER dataset. We explore some simple ways of combining the three approaches. We find that majority voting consistently gives high precision and high F1 across all 4 datasets. Lastly, we implement a system that learns to combine the predictions of SEQ and SpanPred, generating systems that consistently give high recall and high F1 across all 4 datasets. On the GENIA dataset, we find that our learned combiner system significantly boosts F1(+1.2) and recall(+2.1) over the systems being combined. We release all the well-documented code necessary to reproduce all systems at https://github.com/flyingmothman/bionlp.
CLaC at SemEval-2023 Task 2: Comparing Span-Prediction and Sequence-Labeling approaches for NER
May 05, 2023Abstract:This paper summarizes the CLaC submission for the MultiCoNER 2 task which concerns the recognition of complex, fine-grained named entities. We compare two popular approaches for NER, namely Sequence Labeling and Span Prediction. We find that our best Span Prediction system performs slightly better than our best Sequence Labeling system on test data. Moreover, we find that using the larger version of XLM RoBERTa significantly improves performance. Post-competition experiments show that Span Prediction and Sequence Labeling approaches improve when they use special input tokens (<s> and </s>) of XLM-RoBERTa. The code for training all models, preprocessing, and post-processing is available at https://github.com/harshshredding/semeval2023-multiconer-paper.
CLaCLab at SocialDisNER: Using Medical Gazetteers for Named-Entity Recognition of Disease Mentions in Spanish Tweets
Sep 13, 2022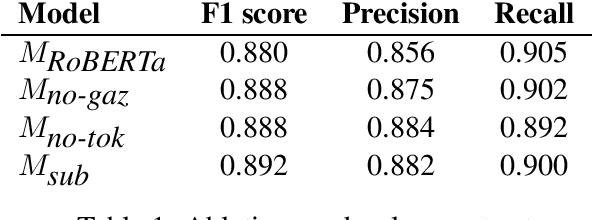
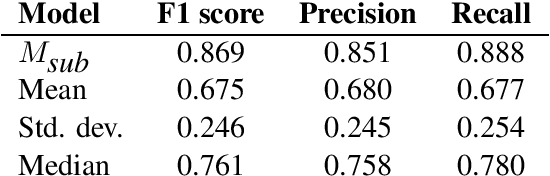
Abstract:This paper summarizes the CLaC submission for SMM4H 2022 Task 10 which concerns the recognition of diseases mentioned in Spanish tweets. Before classifying each token, we encode each token with a transformer encoder using features from Multilingual RoBERTa Large, UMLS gazetteer, and DISTEMIST gazetteer, among others. We obtain a strict F1 score of 0.869, with competition mean of 0.675, standard deviation of 0.245, and median of 0.761.
Multi-label classification for biomedical literature: an overview of the BioCreative VII LitCovid Track for COVID-19 literature topic annotations
Apr 20, 2022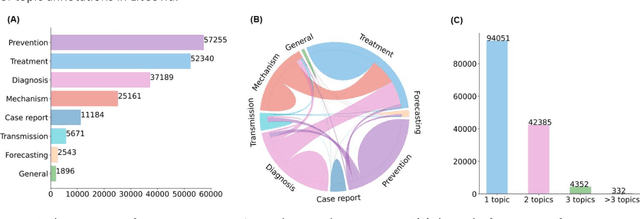
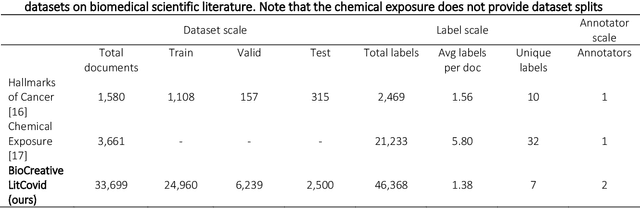
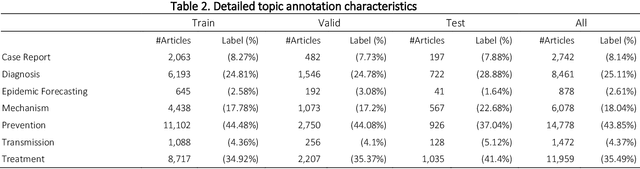
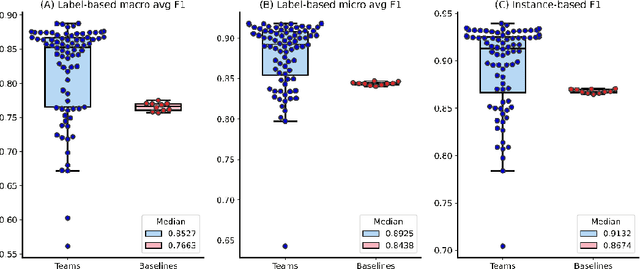
Abstract:The COVID-19 pandemic has been severely impacting global society since December 2019. Massive research has been undertaken to understand the characteristics of the virus and design vaccines and drugs. The related findings have been reported in biomedical literature at a rate of about 10,000 articles on COVID-19 per month. Such rapid growth significantly challenges manual curation and interpretation. For instance, LitCovid is a literature database of COVID-19-related articles in PubMed, which has accumulated more than 200,000 articles with millions of accesses each month by users worldwide. One primary curation task is to assign up to eight topics (e.g., Diagnosis and Treatment) to the articles in LitCovid. Despite the continuing advances in biomedical text mining methods, few have been dedicated to topic annotations in COVID-19 literature. To close the gap, we organized the BioCreative LitCovid track to call for a community effort to tackle automated topic annotation for COVID-19 literature. The BioCreative LitCovid dataset, consisting of over 30,000 articles with manually reviewed topics, was created for training and testing. It is one of the largest multilabel classification datasets in biomedical scientific literature. 19 teams worldwide participated and made 80 submissions in total. Most teams used hybrid systems based on transformers. The highest performing submissions achieved 0.8875, 0.9181, and 0.9394 for macro F1-score, micro F1-score, and instance-based F1-score, respectively. The level of participation and results demonstrate a successful track and help close the gap between dataset curation and method development. The dataset is publicly available via https://ftp.ncbi.nlm.nih.gov/pub/lu/LitCovid/biocreative/ for benchmarking and further development.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge