Rishab Khincha
Uncertainty-Aware Boosted Ensembling in Multi-Modal Settings
Apr 21, 2021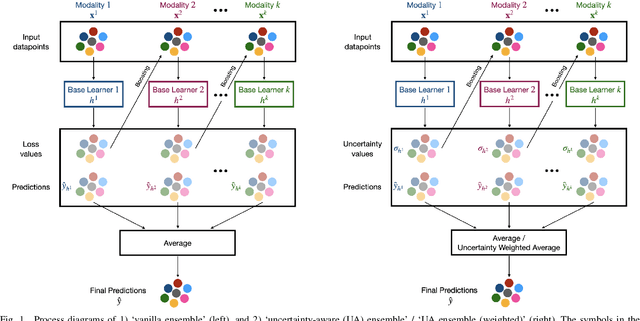
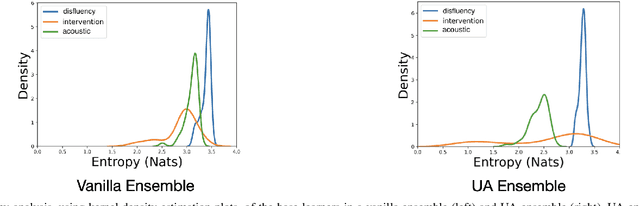
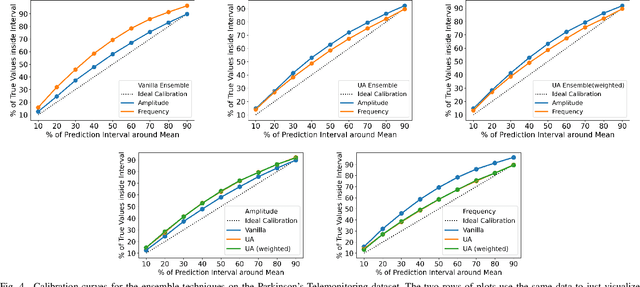
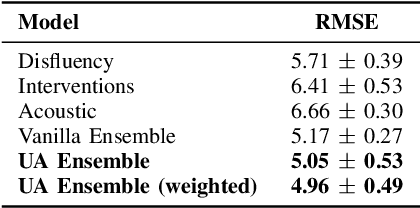
Abstract:Reliability of machine learning (ML) systems is crucial in safety-critical applications such as healthcare, and uncertainty estimation is a widely researched method to highlight the confidence of ML systems in deployment. Sequential and parallel ensemble techniques have shown improved performance of ML systems in multi-modal settings by leveraging the feature sets together. We propose an uncertainty-aware boosting technique for multi-modal ensembling in order to focus on the data points with higher associated uncertainty estimates, rather than the ones with higher loss values. We evaluate this method on healthcare tasks related to Dementia and Parkinson's disease which involve real-world multi-modal speech and text data, wherein our method shows an improved performance. Additional analysis suggests that introducing uncertainty-awareness into the boosted ensembles decreases the overall entropy of the system, making it more robust to heteroscedasticity in the data, as well as better calibrating each of the modalities along with high quality prediction intervals. We open-source our entire codebase at https://github.com/usarawgi911/Uncertainty-aware-boosting
Constructing and Evaluating an Explainable Model for COVID-19 Diagnosis from Chest X-rays
Dec 19, 2020



Abstract:In this paper, our focus is on constructing models to assist a clinician in the diagnosis of COVID-19 patients in situations where it is easier and cheaper to obtain X-ray data than to obtain high-quality images like those from CT scans. Deep neural networks have repeatedly been shown to be capable of constructing highly predictive models for disease detection directly from image data. However, their use in assisting clinicians has repeatedly hit a stumbling block due to their black-box nature. Some of this difficulty can be alleviated if predictions were accompanied by explanations expressed in clinically relevant terms. In this paper, deep neural networks are used to extract domain-specific features(morphological features like ground-glass opacity and disease indications like pneumonia) directly from the image data. Predictions about these features are then used to construct a symbolic model (a decision tree) for the diagnosis of COVID-19 from chest X-rays, accompanied with two kinds of explanations: visual (saliency maps, derived from the neural stage), and textual (logical descriptions, derived from the symbolic stage). A radiologist rates the usefulness of the visual and textual explanations. Our results demonstrate that neural models can be employed usefully in identifying domain-specific features from low-level image data; that textual explanations in terms of clinically relevant features may be useful; and that visual explanations will need to be clinically meaningful to be useful.
Robustness to Missing Features using Hierarchical Clustering with Split Neural Networks
Nov 19, 2020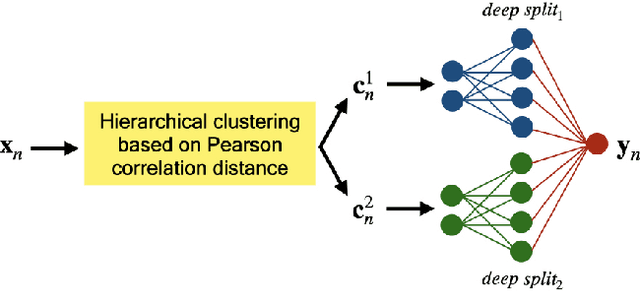
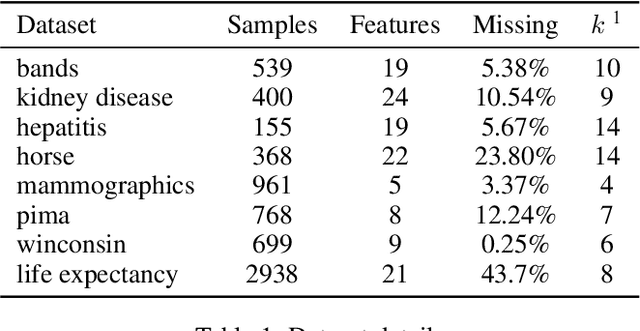
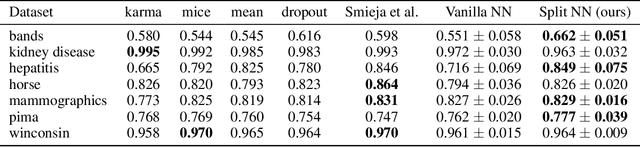
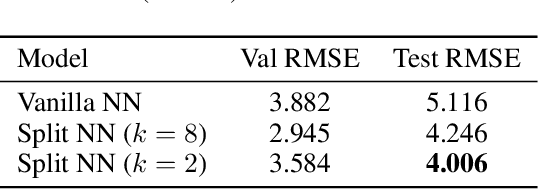
Abstract:The problem of missing data has been persistent for a long time and poses a major obstacle in machine learning and statistical data analysis. Past works in this field have tried using various data imputation techniques to fill in the missing data, or training neural networks (NNs) with the missing data. In this work, we propose a simple yet effective approach that clusters similar input features together using hierarchical clustering and then trains proportionately split neural networks with a joint loss. We evaluate this approach on a series of benchmark datasets and show promising improvements even with simple imputation techniques. We attribute this to learning through clusters of similar features in our model architecture. The source code is available at https://github.com/usarawgi911/Robustness-to-Missing-Features
Uncertainty-Aware Multi-Modal Ensembling for Severity Prediction of Alzheimer's Dementia
Oct 03, 2020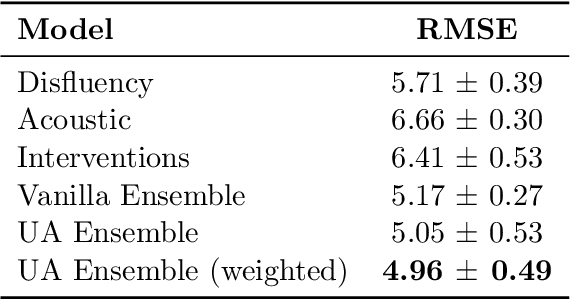
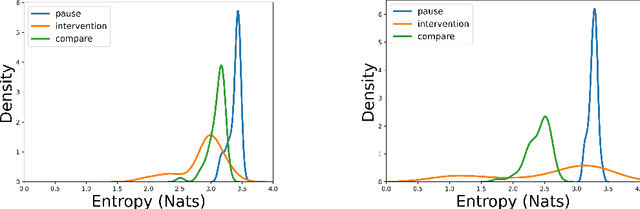

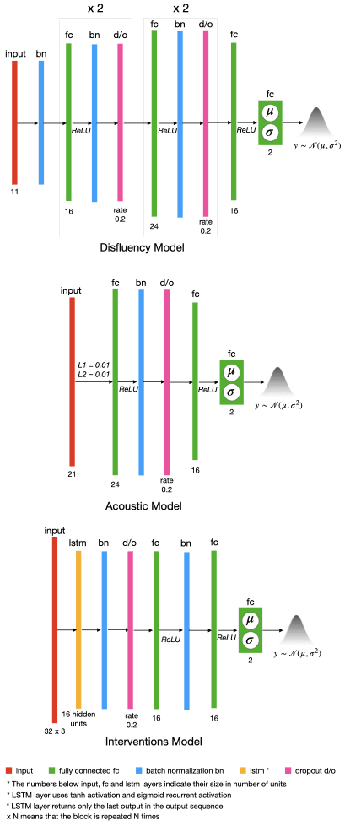
Abstract:Reliability in Neural Networks (NNs) is crucial in safety-critical applications like healthcare, and uncertainty estimation is a widely researched method to highlight the confidence of NNs in deployment. In this work, we propose an uncertainty-aware boosting technique for multi-modal ensembling to predict Alzheimer's Dementia Severity. The propagation of uncertainty across acoustic, cognitive, and linguistic features produces an ensemble system robust to heteroscedasticity in the data. Weighing the different modalities based on the uncertainty estimates, we experiment on the benchmark ADReSS dataset, a subject-independent and balanced dataset, to show that our method outperforms the state-of-the-art methods while also reducing the overall entropy of the system. This work aims to encourage fair and aware models. The source code is available at https://github.com/wazeerzulfikar/alzheimers-dementia
Why have a Unified Predictive Uncertainty? Disentangling it using Deep Split Ensembles
Sep 25, 2020
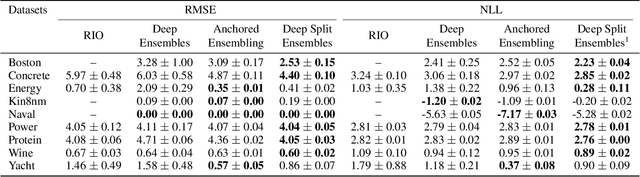
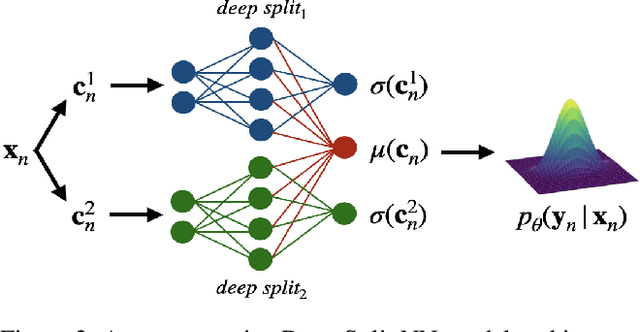
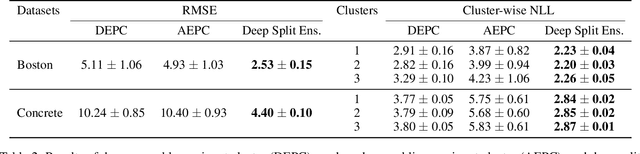
Abstract:Understanding and quantifying uncertainty in black box Neural Networks (NNs) is critical when deployed in real-world settings such as healthcare. Recent works using Bayesian and non-Bayesian methods have shown how a unified predictive uncertainty can be modelled for NNs. Decomposing this uncertainty to disentangle the granular sources of heteroscedasticity in data provides rich information about its underlying causes. We propose a conceptually simple non-Bayesian approach, deep split ensemble, to disentangle the predictive uncertainties using a multivariate Gaussian mixture model. The NNs are trained with clusters of input features, for uncertainty estimates per cluster. We evaluate our approach on a series of benchmark regression datasets, while also comparing with unified uncertainty methods. Extensive analyses using dataset shits and empirical rule highlight our inherently well-calibrated models. Our work further demonstrates its applicability in a multi-modal setting using a benchmark Alzheimer's dataset and also shows how deep split ensembles can highlight hidden modality-specific biases. The minimal changes required to NNs and the training procedure, and the high flexibility to group features into clusters makes it readily deployable and useful. The source code is available at https://github.com/wazeerzulfikar/deep-split-ensembles
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge