Ricarda Fischbach
Distance-based detection of out-of-distribution silent failures for Covid-19 lung lesion segmentation
Aug 05, 2022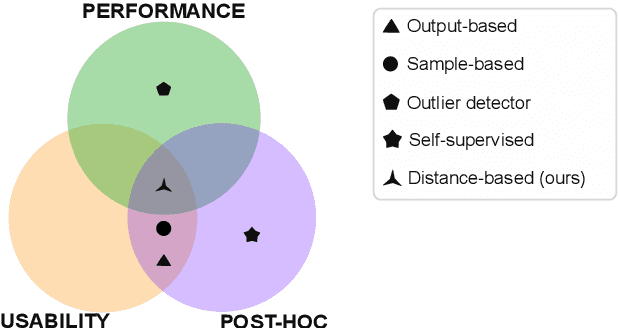
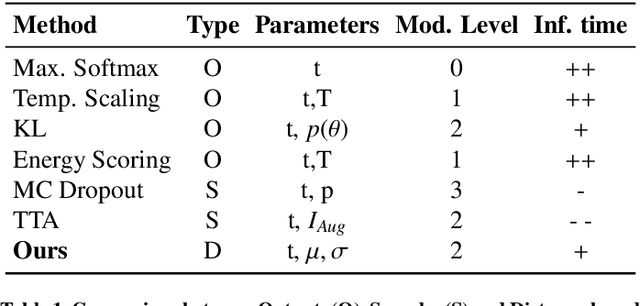
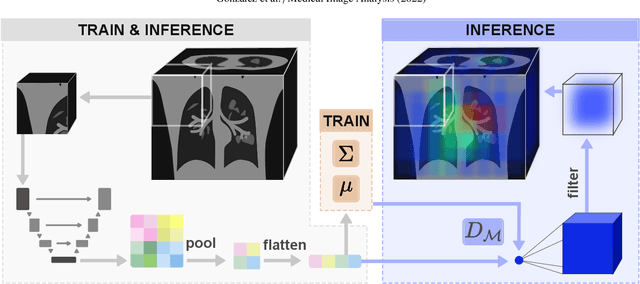
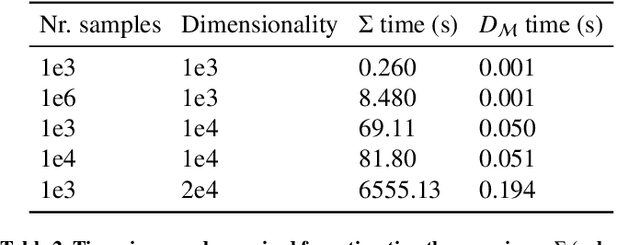
Abstract:Automatic segmentation of ground glass opacities and consolidations in chest computer tomography (CT) scans can potentially ease the burden of radiologists during times of high resource utilisation. However, deep learning models are not trusted in the clinical routine due to failing silently on out-of-distribution (OOD) data. We propose a lightweight OOD detection method that leverages the Mahalanobis distance in the feature space and seamlessly integrates into state-of-the-art segmentation pipelines. The simple approach can even augment pre-trained models with clinically relevant uncertainty quantification. We validate our method across four chest CT distribution shifts and two magnetic resonance imaging applications, namely segmentation of the hippocampus and the prostate. Our results show that the proposed method effectively detects far- and near-OOD samples across all explored scenarios.
Quality monitoring of federated Covid-19 lesion segmentation
Dec 16, 2021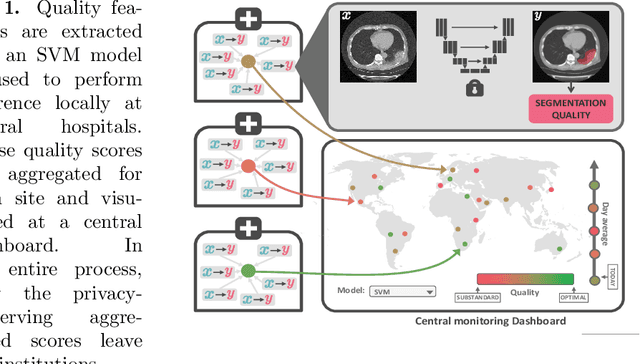
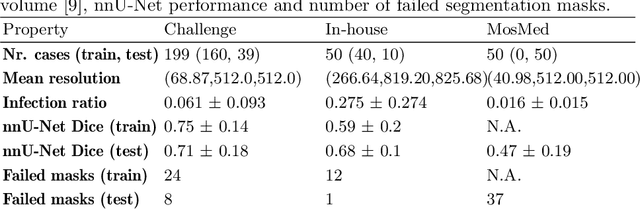
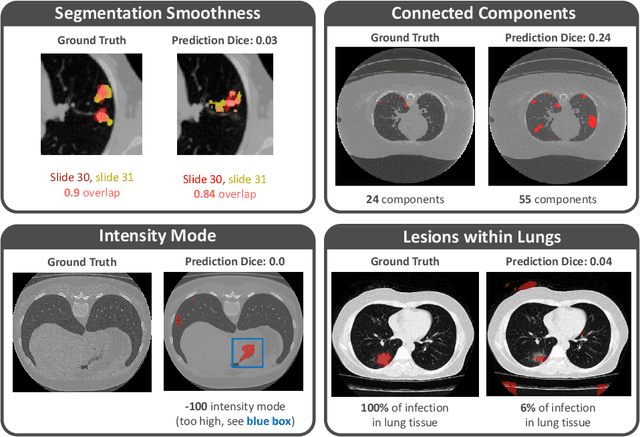
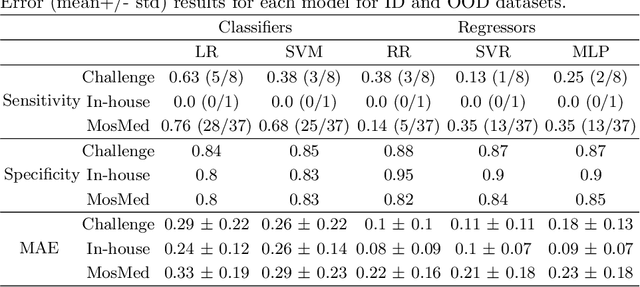
Abstract:Federated Learning is the most promising way to train robust Deep Learning models for the segmentation of Covid-19-related findings in chest CTs. By learning in a decentralized fashion, heterogeneous data can be leveraged from a variety of sources and acquisition protocols whilst ensuring patient privacy. It is, however, crucial to continuously monitor the performance of the model. Yet when it comes to the segmentation of diffuse lung lesions, a quick visual inspection is not enough to assess the quality, and thorough monitoring of all network outputs by expert radiologists is not feasible. In this work, we present an array of lightweight metrics that can be calculated locally in each hospital and then aggregated for central monitoring of a federated system. Our linear model detects over 70% of low-quality segmentations on an out-of-distribution dataset and thus reliably signals a decline in model performance.
Detecting when pre-trained nnU-Net models fail silently for Covid-19 lung lesion segmentation
Jul 14, 2021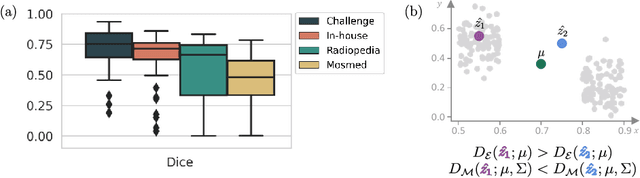
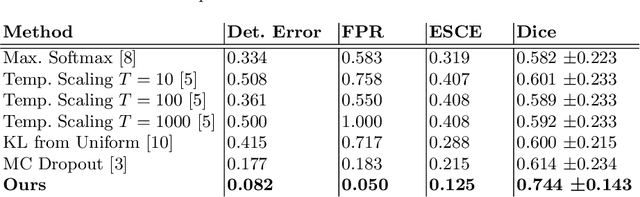
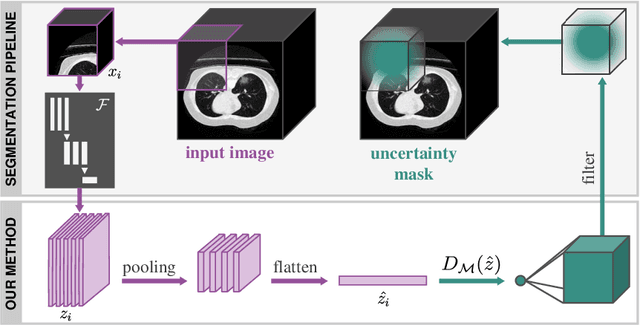
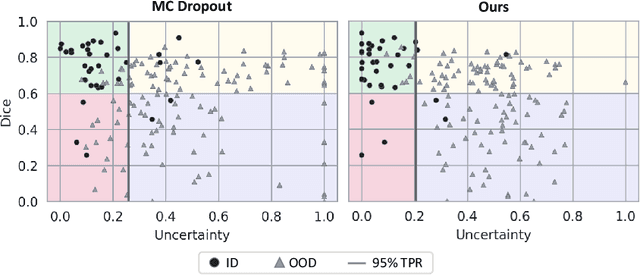
Abstract:Automatic segmentation of lung lesions in computer tomography has the potential to ease the burden of clinicians during the Covid-19 pandemic. Yet predictive deep learning models are not trusted in the clinical routine due to failing silently in out-of-distribution (OOD) data. We propose a lightweight OOD detection method that exploits the Mahalanobis distance in the feature space. The proposed approach can be seamlessly integrated into state-of-the-art segmentation pipelines without requiring changes in model architecture or training procedure, and can therefore be used to assess the suitability of pre-trained models to new data. We validate our method with a patch-based nnU-Net architecture trained with a multi-institutional dataset and find that it effectively detects samples that the model segments incorrectly.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge