René Donner
Unsupervised Identification of Disease Marker Candidates in Retinal OCT Imaging Data
Oct 31, 2018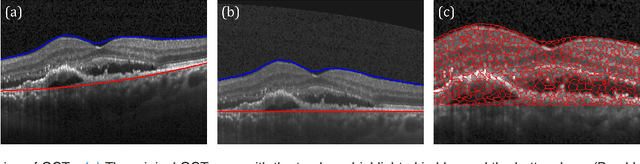
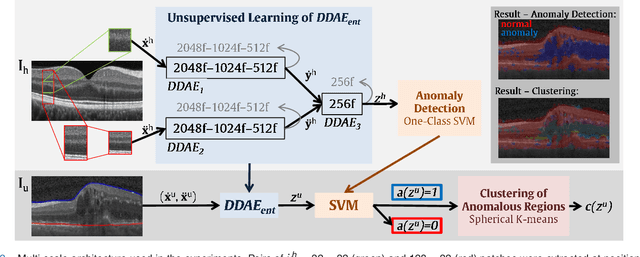
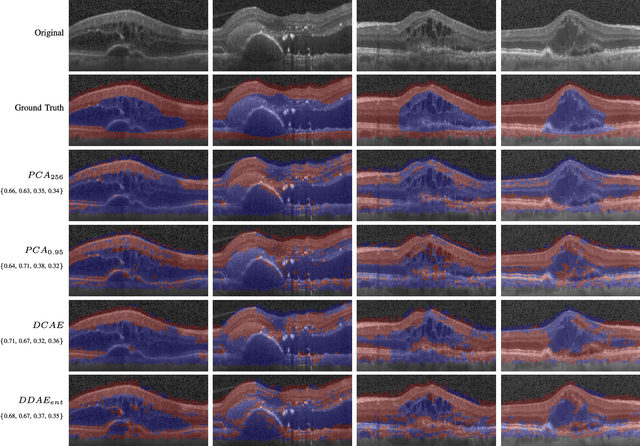
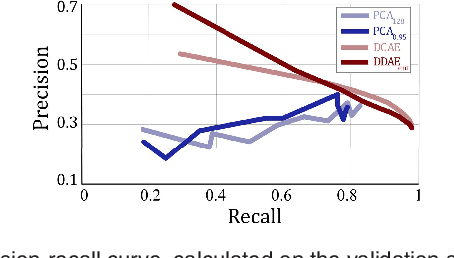
Abstract:The identification and quantification of markers in medical images is critical for diagnosis, prognosis, and disease management. Supervised machine learning enables the detection and exploitation of findings that are known a priori after annotation of training examples by experts. However, supervision does not scale well, due to the amount of necessary training examples, and the limitation of the marker vocabulary to known entities. In this proof-of-concept study, we propose unsupervised identification of anomalies as candidates for markers in retinal Optical Coherence Tomography (OCT) imaging data without a constraint to a priori definitions. We identify and categorize marker candidates occurring frequently in the data, and demonstrate that these markers show predictive value in the task of detecting disease. A careful qualitative analysis of the identified data driven markers reveals how their quantifiable occurrence aligns with our current understanding of disease course, in early- and late age-related macular degeneration (AMD) patients. A multi-scale deep denoising autoencoder is trained on healthy images, and a one-class support vector machine identifies anomalies in new data. Clustering in the anomalies identifies stable categories. Using these markers to classify healthy-, early AMD- and late AMD cases yields an accuracy of 81.40%. In a second binary classification experiment on a publicly available data set (healthy vs. intermediate AMD) the model achieves an area under the ROC curve of 0.944.
Identifying and Categorizing Anomalies in Retinal Imaging Data
Dec 02, 2016
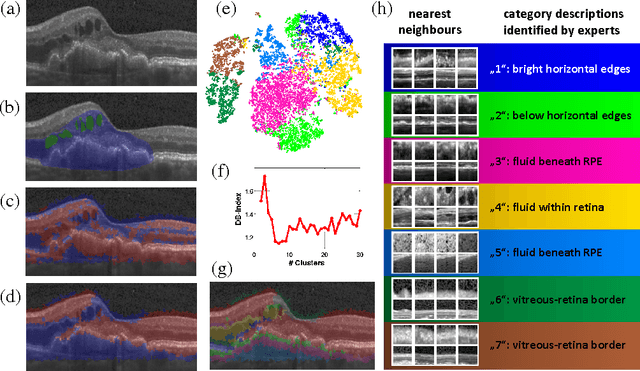
Abstract:The identification and quantification of markers in medical images is critical for diagnosis, prognosis and management of patients in clinical practice. Supervised- or weakly supervised training enables the detection of findings that are known a priori. It does not scale well, and a priori definition limits the vocabulary of markers to known entities reducing the accuracy of diagnosis and prognosis. Here, we propose the identification of anomalies in large-scale medical imaging data using healthy examples as a reference. We detect and categorize candidates for anomaly findings untypical for the observed data. A deep convolutional autoencoder is trained on healthy retinal images. The learned model generates a new feature representation, and the distribution of healthy retinal patches is estimated by a one-class support vector machine. Results demonstrate that we can identify pathologic regions in images without using expert annotations. A subsequent clustering categorizes findings into clinically meaningful classes. In addition the learned features outperform standard embedding approaches in a classification task.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge