Reihaneh Hassanzadeh
Hierarchical Spatio-Temporal State-Space Modeling for fMRI Analysis
Aug 23, 2024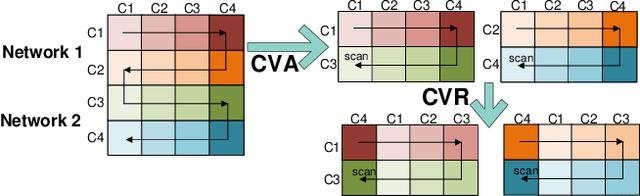
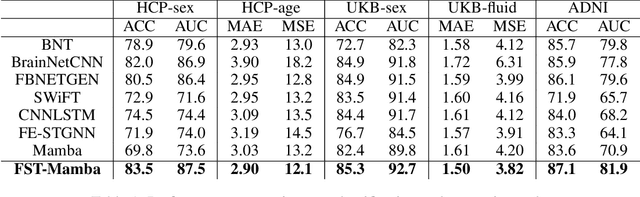
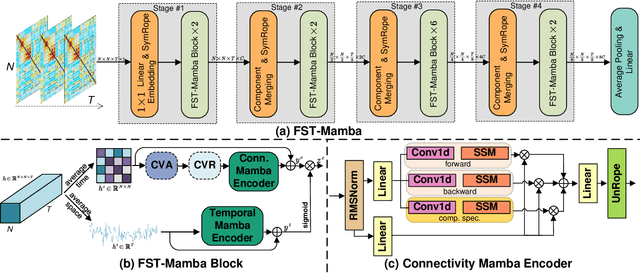

Abstract:Recent advances in deep learning structured state space models, especially the Mamba architecture, have demonstrated remarkable performance improvements while maintaining linear complexity. In this study, we introduce functional spatiotemporal Mamba (FST-Mamba), a Mamba-based model designed for discovering neurological biomarkers using functional magnetic resonance imaging (fMRI). We focus on dynamic functional network connectivity (dFNC) derived from fMRI and propose a hierarchical spatiotemporal Mamba-based network that processes spatial and temporal information separately using Mamba-based encoders. Leveraging the topological uniqueness of the FNC matrix, we introduce a component-wise varied-scale aggregation (CVA) mechanism to aggregate connectivity across individual components within brain networks, enabling the model to capture both inter-component and inter-network information. To better handle the FNC data, we develop a new component-specific scanning order. Additionally, we propose symmetric rotary position encoding (SymRope) to encode the relative positions of each functional connection while considering the symmetric nature of the FNC matrix. Experimental results demonstrate significant improvements in the proposed FST-Mamba model on various brain-based classification and regression tasks. Our work reveals the substantial potential of attention-free sequence modeling in brain discovery.
Cross-Modality Translation with Generative Adversarial Networks to Unveil Alzheimer's Disease Biomarkers
May 08, 2024Abstract:Generative approaches for cross-modality transformation have recently gained significant attention in neuroimaging. While most previous work has focused on case-control data, the application of generative models to disorder-specific datasets and their ability to preserve diagnostic patterns remain relatively unexplored. Hence, in this study, we investigated the use of a generative adversarial network (GAN) in the context of Alzheimer's disease (AD) to generate functional network connectivity (FNC) and T1-weighted structural magnetic resonance imaging data from each other. We employed a cycle-GAN to synthesize data in an unpaired data transition and enhanced the transition by integrating weak supervision in cases where paired data were available. Our findings revealed that our model could offer remarkable capability, achieving a structural similarity index measure (SSIM) of $0.89 \pm 0.003$ for T1s and a correlation of $0.71 \pm 0.004$ for FNCs. Moreover, our qualitative analysis revealed similar patterns between generated and actual data when comparing AD to cognitively normal (CN) individuals. In particular, we observed significantly increased functional connectivity in cerebellar-sensory motor and cerebellar-visual networks and reduced connectivity in cerebellar-subcortical, auditory-sensory motor, sensory motor-visual, and cerebellar-cognitive control networks. Additionally, the T1 images generated by our model showed a similar pattern of atrophy in the hippocampal and other temporal regions of Alzheimer's patients.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge