Philipp Rosendahl
Automated Quality Control of Vacuum Insulated Glazing by Convolutional Neural Network Image Classification
Oct 15, 2021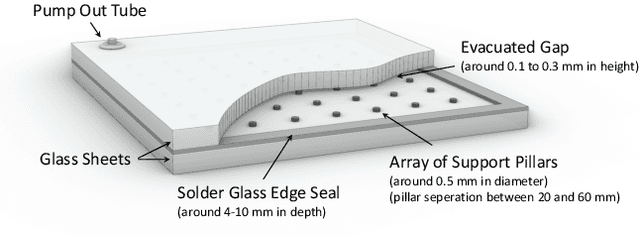
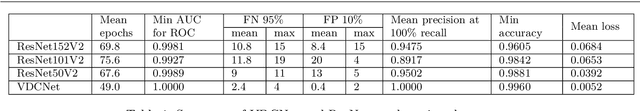
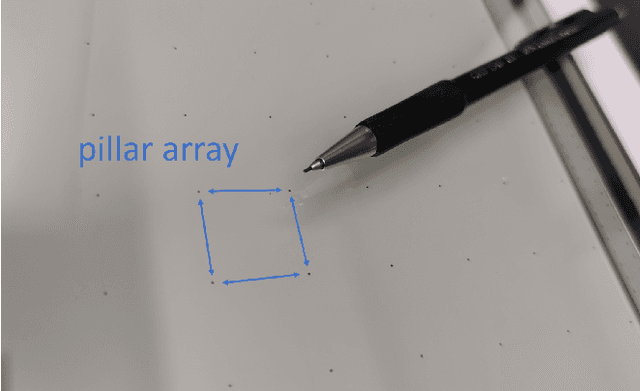
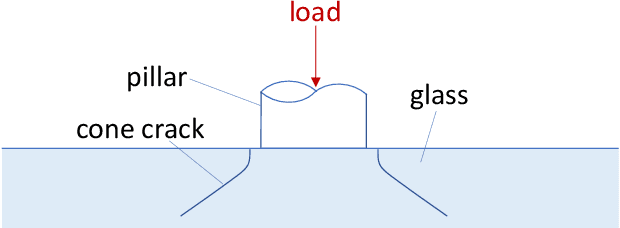
Abstract:Vacuum Insulated Glazing (VIG) is a highly thermally insulating window technology, which boasts an extremely thin profile and lower weight as compared to gas-filled insulated glazing units of equivalent performance. The VIG is a double-pane configuration with a submillimeter vacuum gap between the panes and therefore under constant atmospheric pressure over their service life. Small pillars are positioned between the panes to maintain the gap, which can damage the glass reducing the lifetime of the VIG unit. To efficiently assess any surface damage on the glass, an automated damage detection system is highly desirable. For the purpose of classifying the damage, we have developed, trained, and tested a deep learning computer vision system using convolutional neural networks. The classification model flawlessly classified the test dataset with an area under the curve (AUC) for the receiver operating characteristic (ROC) of 100%. We have automatically cropped the images down to their relevant information by using Faster-RCNN to locate the position of the pillars. We employ the state-of-the-art methods Grad-CAM and Score-CAM of explainable Artificial Intelligence (XAI) to provide an understanding of the internal mechanisms and were able to show that our classifier outperforms ResNet50V2 for identification of crack locations and geometry. The proposed methods can therefore be used to detect systematic defects even without large amounts of training data. Further analyses of our model's predictive capabilities demonstrates its superiority over state-of-the-art models (ResNet50V2, ResNet101V2 and ResNet152V2) in terms of convergence speed, accuracy, precision at 100% recall and AUC for ROC.
Cell Mechanics Based Computational Classification of Red Blood Cells Via Machine Intelligence Applied to Morpho-Rheological Markers
Mar 02, 2020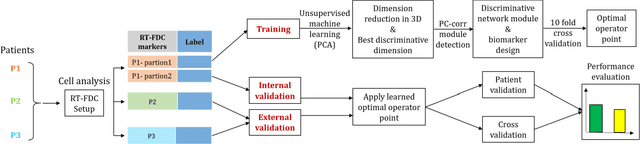
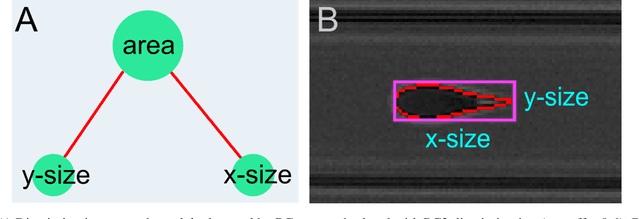
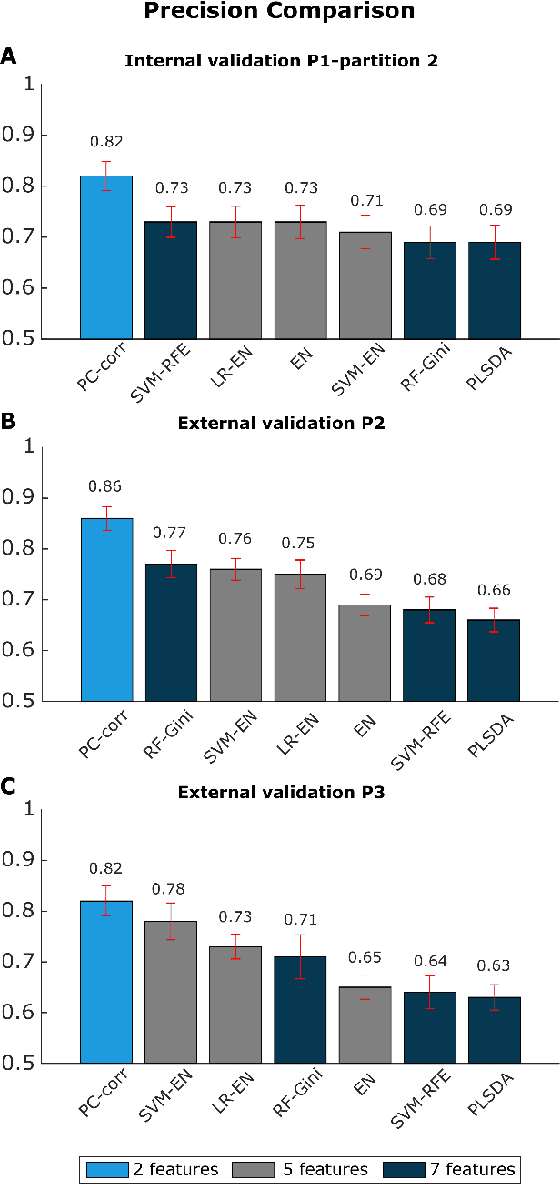
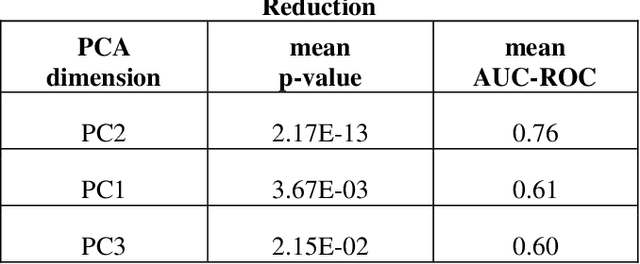
Abstract:Despite fluorescent cell-labelling being widely employed in biomedical studies, some of its drawbacks are inevitable, with unsuitable fluorescent probes or probes inducing a functional change being the main limitations. Consequently, the demand for and development of label-free methodologies to classify cells is strong and its impact on precision medicine is relevant. Towards this end, high-throughput techniques for cell mechanical phenotyping have been proposed to get a multidimensional biophysical characterization of single cells. With this motivation, our goal here is to investigate the extent to which an unsupervised machine learning methodology, which is applied exclusively on morpho-rheological markers obtained by real-time deformability and fluorescence cytometry (RT-FDC), can address the difficult task of providing label-free discrimination of reticulocytes from mature red blood cells. We focused on this problem, since the characterization of reticulocytes (their percentage and cellular features) in the blood is vital in multiple human disease conditions, especially bone-marrow disorders such as anemia and leukemia. Our approach reports promising label-free results in the classification of reticulocytes from mature red blood cells, and it represents a step forward in the development of high-throughput morpho-rheological-based methodologies for the computational categorization of single cells. Besides, our methodology can be an alternative but also a complementary method to integrate with existing cell-labelling techniques.
* 13 pages, 3 figures, 4 tables
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge