Orlando Aristizabal
Deep Mouse: An End-to-end Auto-context Refinement Framework for Brain Ventricle and Body Segmentation in Embryonic Mice Ultrasound Volumes
Oct 30, 2019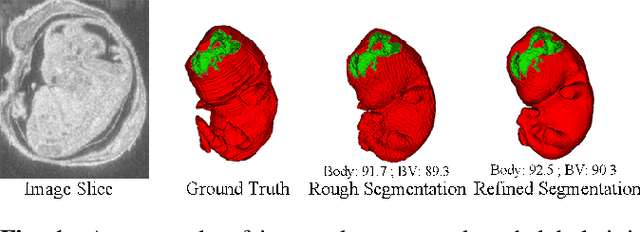
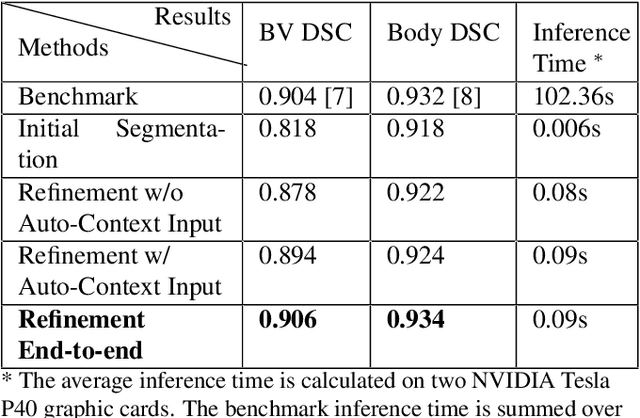
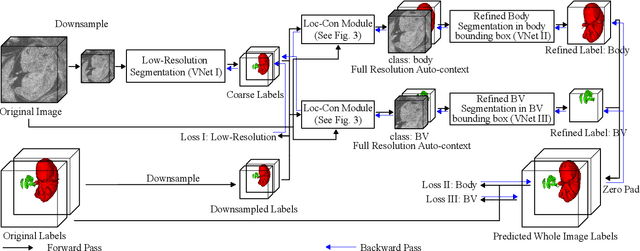
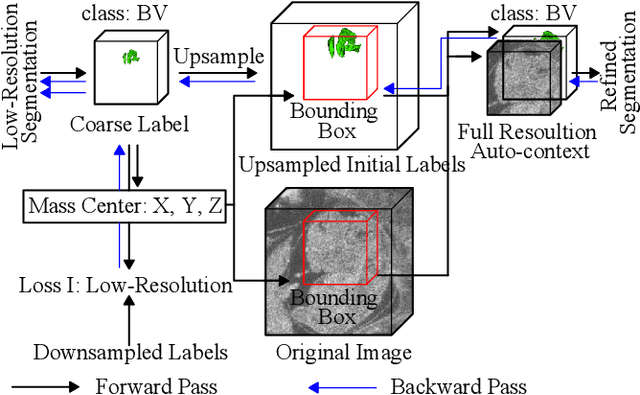
Abstract:High-frequency ultrasound (HFU) is well suited for imaging embryonic mice due to its noninvasive and real-time characteristics. However, manual segmentation of the brain ventricles (BVs) and body requires substantial time and expertise. This work proposes a novel deep learning based end-to-end auto-context refinement framework, consisting of two stages. The first stage produces a low resolution segmentation of the BV and body simultaneously. The resulting probability map for each object (BV or body) is then used to crop a region of interest (ROI) around the target object in both the original image and the probability map to provide context to the refinement segmentation network. Joint training of the two stages provides significant improvement in Dice Similarity Coefficient (DSC) over using only the first stage (0.818 to 0.906 for the BV, and 0.919 to 0.934 for the body). The proposed method significantly reduces the inference time (102.36 to 0.09 s/volume around 1000x faster) while slightly improves the segmentation accuracy over the previous methods using slide-window approaches.
Automatic Mouse Embryo Brain Ventricle & Body Segmentation and Mutant Classification From Ultrasound Data Using Deep Learning
Sep 23, 2019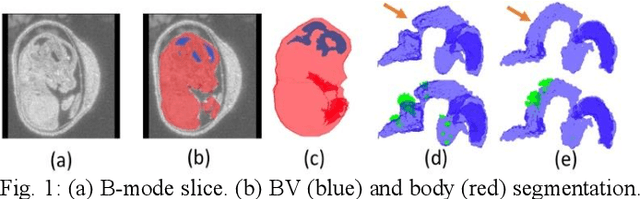
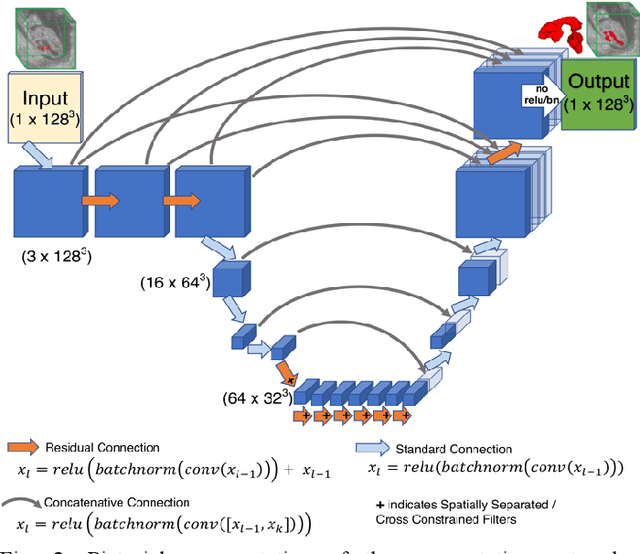
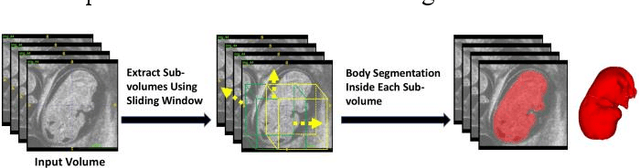
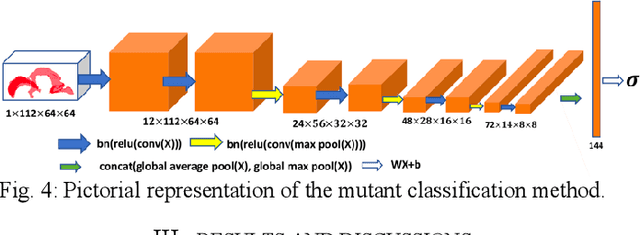
Abstract:High-frequency ultrasound (HFU) is well suited for imaging embryonic mice in vivo because it is non-invasive and real-time. Manual segmentation of the brain ventricles (BVs) and whole body from 3D HFU images is time-consuming and requires specialized training. This paper presents a deep-learning-based segmentation pipeline which automates several time-consuming, repetitive tasks currently performed to study genetic mutations in developing mouse embryos. Namely, the pipeline accurately segments the BV and body regions in 3D HFU images of mouse embryos, despite significant challenges due to position and shape variation of the embryos, as well as imaging artifacts. Based on the BV segmentation, a 3D convolutional neural network (CNN) is further trained to detect embryos with the Engrailed-1 (En1) mutation. The algorithms achieve 0.896 and 0.925 Dice Similarity Coefficient (DSC) for BV and body segmentation, respectively, and 95.8% accuracy on mutant classification. Through gradient based interrogation and visualization of the trained classifier, it is demonstrated that the model focuses on the morphological structures known to be affected by the En1 mutation.
Deep BV: A Fully Automated System for Brain Ventricle Localization and Segmentation in 3D Ultrasound Images of Embryonic Mice
Nov 05, 2018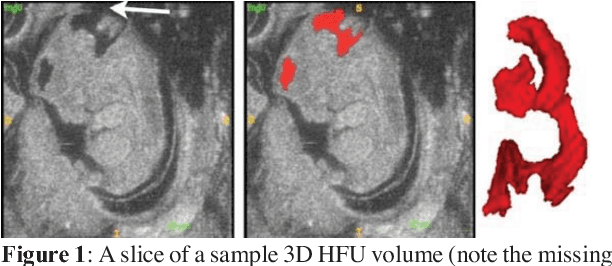
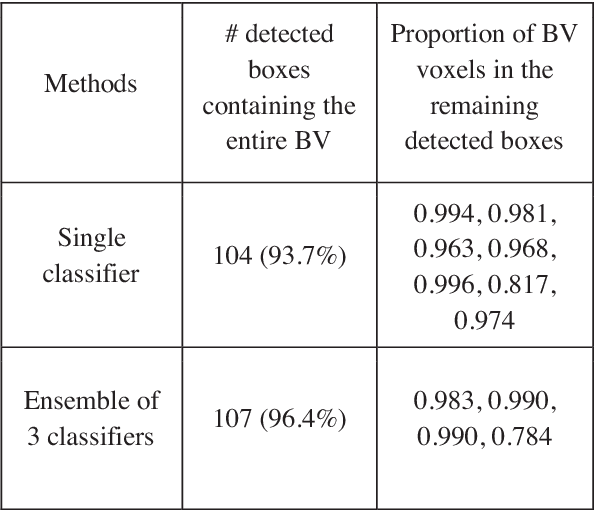
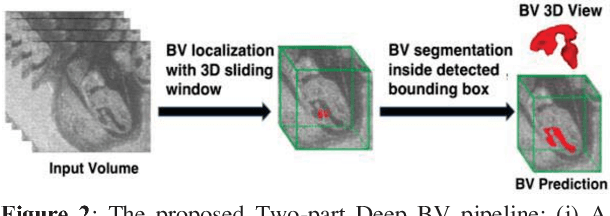
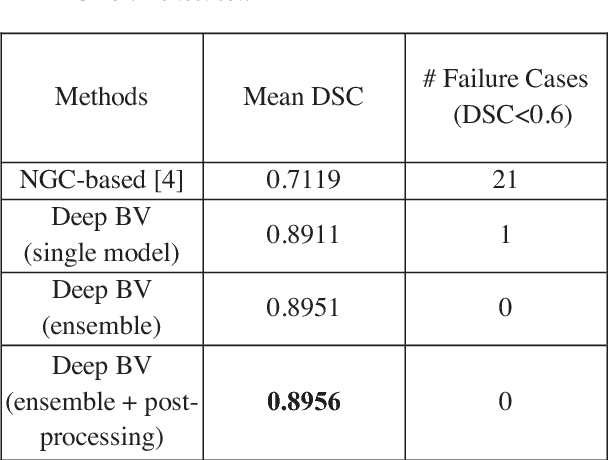
Abstract:Volumetric analysis of brain ventricle (BV) structure is a key tool in the study of central nervous system development in embryonic mice. High-frequency ultrasound (HFU) is the only non-invasive, real-time modality available for rapid volumetric imaging of embryos in utero. However, manual segmentation of the BV from HFU volumes is tedious, time-consuming, and requires specialized expertise. In this paper, we propose a novel deep learning based BV segmentation system for whole-body HFU images of mouse embryos. Our fully automated system consists of two modules: localization and segmentation. It first applies a volumetric convolutional neural network on a 3D sliding window over the entire volume to identify a 3D bounding box containing the entire BV. It then employs a fully convolutional network to segment the detected bounding box into BV and background. The system achieves a Dice Similarity Coefficient (DSC) of 0.8956 for BV segmentation on an unseen 111 HFU volume test set surpassing the previous state-of-the-art method (DSC of 0.7119) by a margin of 25%.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge