Automatic Mouse Embryo Brain Ventricle & Body Segmentation and Mutant Classification From Ultrasound Data Using Deep Learning
Paper and Code
Sep 23, 2019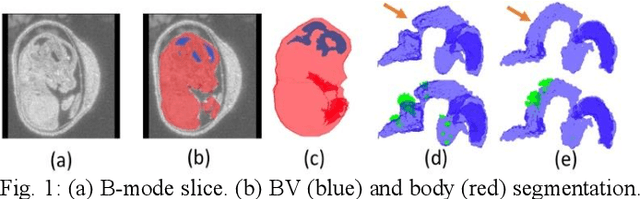
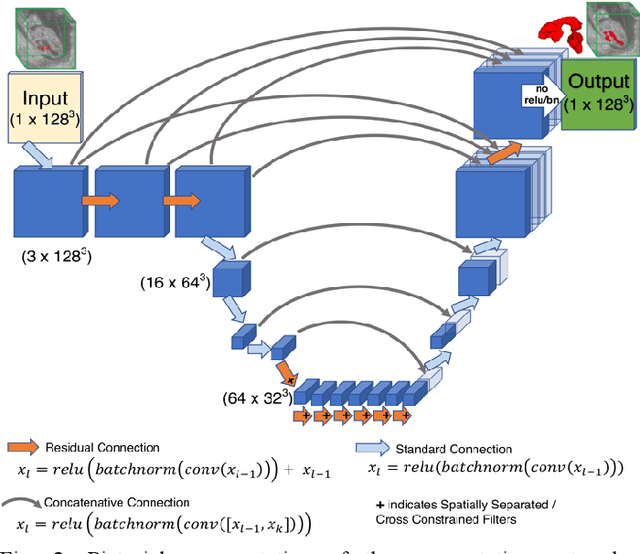
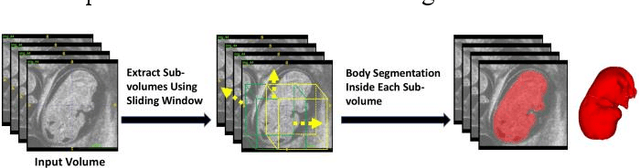
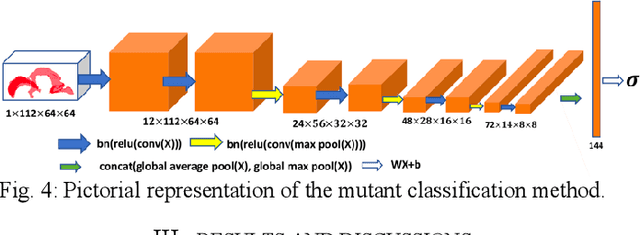
High-frequency ultrasound (HFU) is well suited for imaging embryonic mice in vivo because it is non-invasive and real-time. Manual segmentation of the brain ventricles (BVs) and whole body from 3D HFU images is time-consuming and requires specialized training. This paper presents a deep-learning-based segmentation pipeline which automates several time-consuming, repetitive tasks currently performed to study genetic mutations in developing mouse embryos. Namely, the pipeline accurately segments the BV and body regions in 3D HFU images of mouse embryos, despite significant challenges due to position and shape variation of the embryos, as well as imaging artifacts. Based on the BV segmentation, a 3D convolutional neural network (CNN) is further trained to detect embryos with the Engrailed-1 (En1) mutation. The algorithms achieve 0.896 and 0.925 Dice Similarity Coefficient (DSC) for BV and body segmentation, respectively, and 95.8% accuracy on mutant classification. Through gradient based interrogation and visualization of the trained classifier, it is demonstrated that the model focuses on the morphological structures known to be affected by the En1 mutation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge