Mohammad Hossein Zangooei
Handling of uncertainty in medical data using machine learning and probability theory techniques: A review of 30 years
Aug 23, 2020
Abstract:Understanding data and reaching valid conclusions are of paramount importance in the present era of big data. Machine learning and probability theory methods have widespread application for this purpose in different fields. One critically important yet less explored aspect is how data and model uncertainties are captured and analyzed. Proper quantification of uncertainty provides valuable information for optimal decision making. This paper reviewed related studies conducted in the last 30 years (from 1991 to 2020) in handling uncertainties in medical data using probability theory and machine learning techniques. Medical data is more prone to uncertainty due to the presence of noise in the data. So, it is very important to have clean medical data without any noise to get accurate diagnosis. The sources of noise in the medical data need to be known to address this issue. Based on the medical data obtained by the physician, diagnosis of disease, and treatment plan are prescribed. Hence, the uncertainty is growing in healthcare and there is limited knowledge to address these problems. We have little knowledge about the optimal treatment methods as there are many sources of uncertainty in medical science. Our findings indicate that there are few challenges to be addressed in handling the uncertainty in medical raw data and new models. In this work, we have summarized various methods employed to overcome this problem. Nowadays, application of novel deep learning techniques to deal such uncertainties have significantly increased.
U-Net Based Architecture for an Improved Multiresolution Segmentation in Medical Images
Jul 17, 2020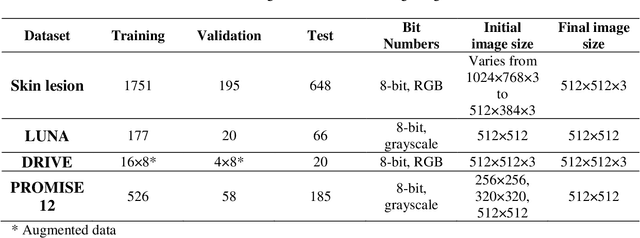
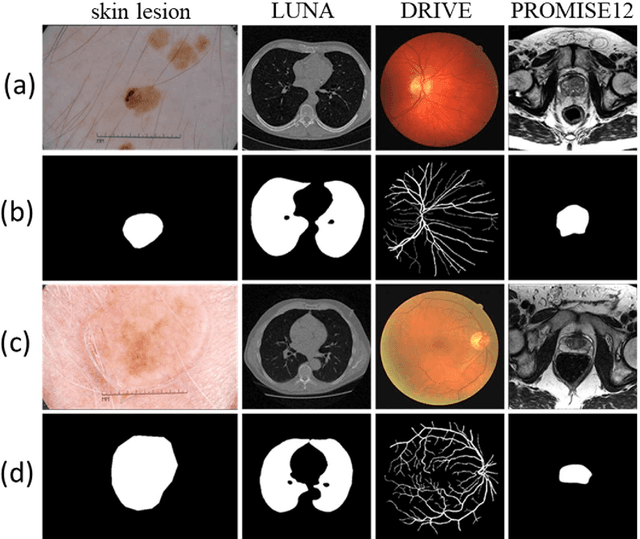
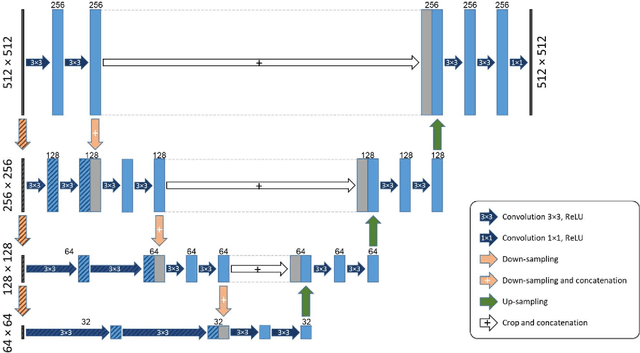
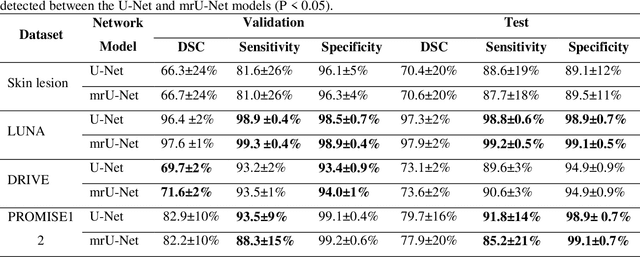
Abstract:Purpose: Manual medical image segmentation is an exhausting and time-consuming task along with high inter-observer variability. In this study, our objective is to improve the multi-resolution image segmentation performance of U-Net architecture. Approach: We have proposed a fully convolutional neural network for image segmentation in a multi-resolution framework. We used U-Net as the base architecture and modified that to improve its image segmentation performance. In the proposed architecture (mrU-Net), the input image and its down-sampled versions were used as the network inputs. We added more convolution layers to extract features directly from the down-sampled images. We trained and tested the network on four different medical datasets, including skin lesion photos, lung computed tomography (CT) images (LUNA dataset), retina images (DRIVE dataset), and prostate magnetic resonance (MR) images (PROMISE12 dataset). We compared the performance of mrU-Net to U-Net under similar training and testing conditions. Results: Comparing the results to manual segmentation labels, mrU-Net achieved average Dice similarity coefficients of 70.6%, 97.9%, 73.6%, and 77.9% for the skin lesion, LUNA, DRIVE, and PROMISE12 segmentation, respectively. For the skin lesion, LUNA, and DRIVE datasets, mrU-Net outperformed U-Net with significantly higher accuracy and for the PROMISE12 dataset, both networks achieved similar accuracy. Furthermore, using mrU-Net led to a faster training rate on LUNA and DRIVE datasets when compared to U-Net. Conclusions: The striking feature of the proposed architecture is its higher capability in extracting image-derived features compared to U-Net. mrU-Net illustrated a faster training rate and slightly more accurate image segmentation compared to U-Net.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge