Michael S. Hansen
Imaging Transformer for MRI Denoising: a Scalable Model Architecture that enables SNR << 1 Imaging
Apr 13, 2025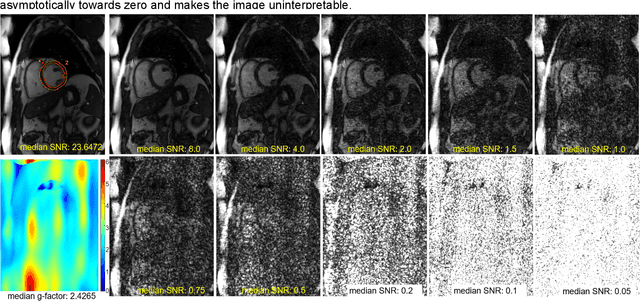

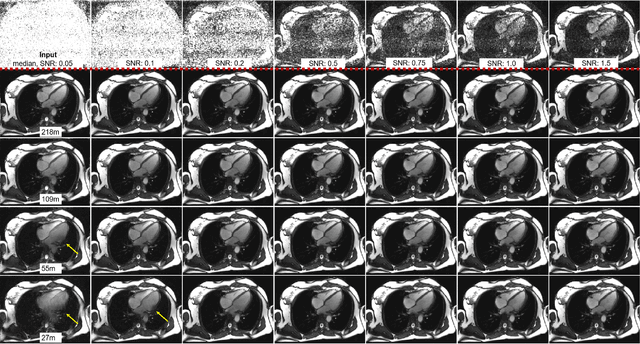
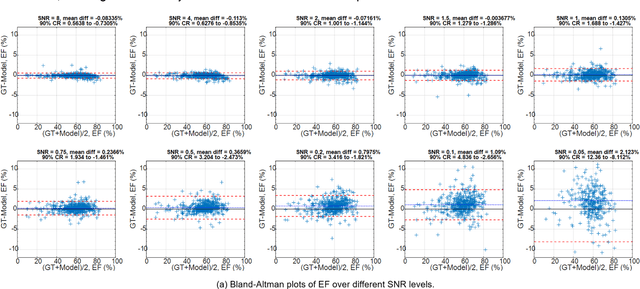
Abstract:Purpose: To propose a flexible and scalable imaging transformer (IT) architecture with three attention modules for multi-dimensional imaging data and apply it to MRI denoising with very low input SNR. Methods: Three independent attention modules were developed: spatial local, spatial global, and frame attentions. They capture long-range signal correlation and bring back the locality of information in images. An attention-cell-block design processes 5D tensors ([B, C, F, H, W]) for 2D, 2D+T, and 3D image data. A High Resolution (HRNet) backbone was built to hold IT blocks. Training dataset consists of 206,677 cine series and test datasets had 7,267 series. Ten input SNR levels from 0.05 to 8.0 were tested. IT models were compared to seven convolutional and transformer baselines. To test scalability, four IT models 27m to 218m parameters were trained. Two senior cardiologists reviewed IT model outputs from which the EF was measured and compared against the ground-truth. Results: IT models significantly outperformed other models over the tested SNR levels. The performance gap was most prominent at low SNR levels. The IT-218m model had the highest SSIM and PSNR, restoring good image quality and anatomical details even at SNR 0.2. Two experts agreed at this SNR or above, the IT model output gave the same clinical interpretation as the ground-truth. The model produced images that had accurate EF measurements compared to ground-truth values. Conclusions: Imaging transformer model offers strong performance, scalability, and versatility for MR denoising. It recovers image quality suitable for confident clinical reading and accurate EF measurement, even at very low input SNR of 0.2.
A Path Towards Clinical Adaptation of Accelerated MRI
Aug 26, 2022



Abstract:Accelerated MRI reconstructs images of clinical anatomies from sparsely sampled signal data to reduce patient scan times. While recent works have leveraged deep learning to accomplish this task, such approaches have often only been explored in simulated environments where there is no signal corruption or resource limitations. In this work, we explore augmentations to neural network MRI image reconstructors to enhance their clinical relevancy. Namely, we propose a ConvNet model for detecting sources of image artifacts that achieves a classifer $F_2$ score of $79.1\%$. We also demonstrate that training reconstructors on MR signal data with variable acceleration factors can improve their average performance during a clinical patient scan by up to $2\%$. We offer a loss function to overcome catastrophic forgetting when models learn to reconstruct MR images of multiple anatomies and orientations. Finally, we propose a method for using simulated phantom data to pre-train reconstructors in situations with limited clinically acquired datasets and compute capabilities. Our results provide a potential path forward for clinical adaptation of accelerated MRI.
End-to-End AI-based MRI Reconstruction and Lesion Detection Pipeline for Evaluation of Deep Learning Image Reconstruction
Sep 23, 2021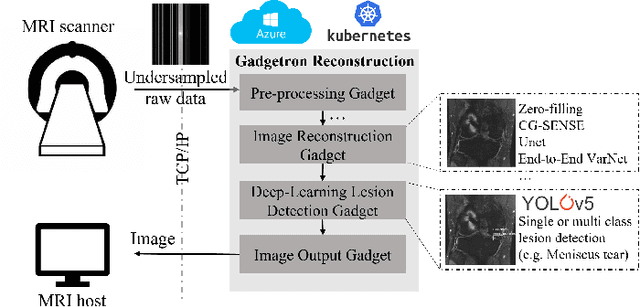

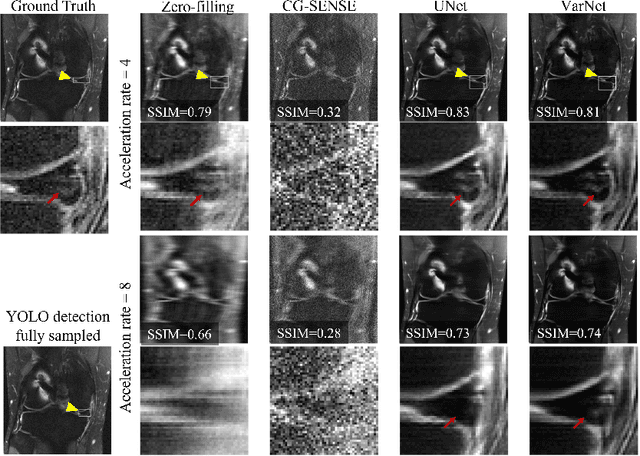

Abstract:Deep learning techniques have emerged as a promising approach to highly accelerated MRI. However, recent reconstruction challenges have shown several drawbacks in current deep learning approaches, including the loss of fine image details even using models that perform well in terms of global quality metrics. In this study, we propose an end-to-end deep learning framework for image reconstruction and pathology detection, which enables a clinically aware evaluation of deep learning reconstruction quality. The solution is demonstrated for a use case in detecting meniscal tears on knee MRI studies, ultimately finding a loss of fine image details with common reconstruction methods expressed as a reduced ability to detect important pathology like meniscal tears. Despite the common practice of quantitative reconstruction methodology evaluation with metrics such as SSIM, impaired pathology detection as an automated pathology-based reconstruction evaluation approach suggests existing quantitative methods do not capture clinically important reconstruction outcomes.
fastMRI+: Clinical Pathology Annotations for Knee and Brain Fully Sampled Multi-Coil MRI Data
Sep 14, 2021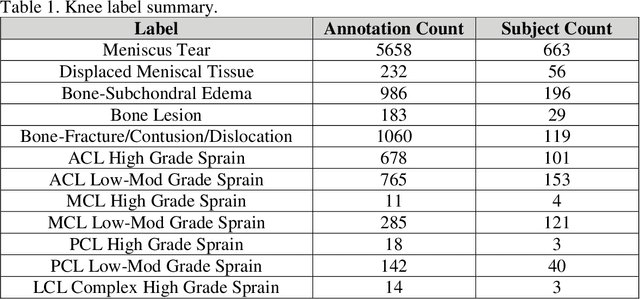
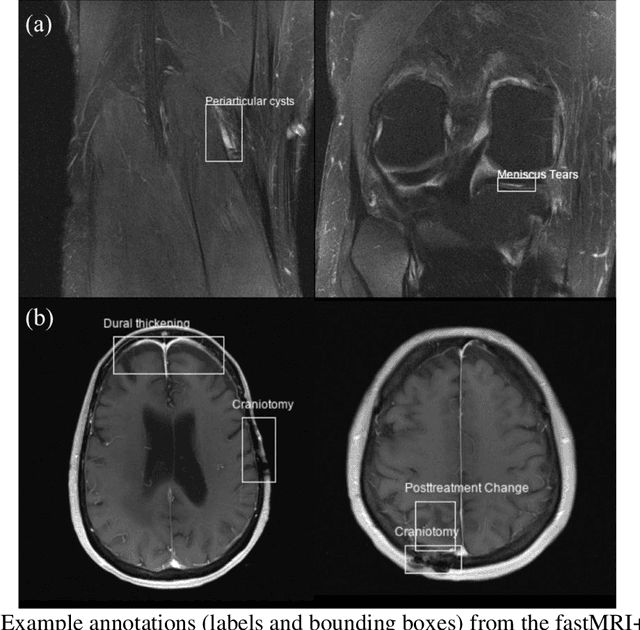
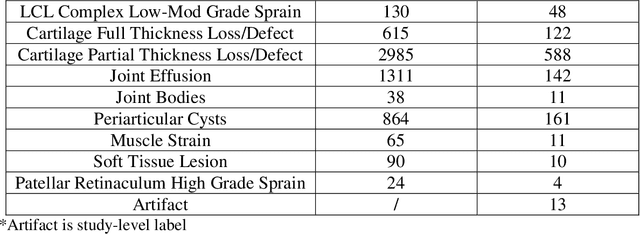
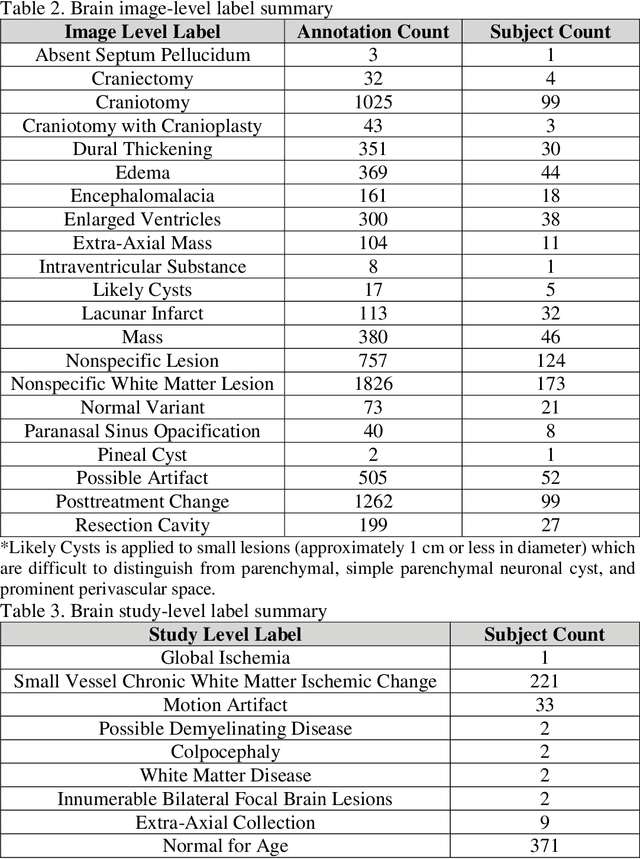
Abstract:Improving speed and image quality of Magnetic Resonance Imaging (MRI) via novel reconstruction approaches remains one of the highest impact applications for deep learning in medical imaging. The fastMRI dataset, unique in that it contains large volumes of raw MRI data, has enabled significant advances in accelerating MRI using deep learning-based reconstruction methods. While the impact of the fastMRI dataset on the field of medical imaging is unquestioned, the dataset currently lacks clinical expert pathology annotations, critical to addressing clinically relevant reconstruction frameworks and exploring important questions regarding rendering of specific pathology using such novel approaches. This work introduces fastMRI+, which consists of 16154 subspecialist expert bounding box annotations and 13 study-level labels for 22 different pathology categories on the fastMRI knee dataset, and 7570 subspecialist expert bounding box annotations and 643 study-level labels for 30 different pathology categories for the fastMRI brain dataset. The fastMRI+ dataset is open access and aims to support further research and advancement of medical imaging in MRI reconstruction and beyond.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge