Michael Bach
A Multi-Centric Anthropomorphic 3D CT Phantom-Based Benchmark Dataset for Harmonization
Jul 02, 2025Abstract:Artificial intelligence (AI) has introduced numerous opportunities for human assistance and task automation in medicine. However, it suffers from poor generalization in the presence of shifts in the data distribution. In the context of AI-based computed tomography (CT) analysis, significant data distribution shifts can be caused by changes in scanner manufacturer, reconstruction technique or dose. AI harmonization techniques can address this problem by reducing distribution shifts caused by various acquisition settings. This paper presents an open-source benchmark dataset containing CT scans of an anthropomorphic phantom acquired with various scanners and settings, which purpose is to foster the development of AI harmonization techniques. Using a phantom allows fixing variations attributed to inter- and intra-patient variations. The dataset includes 1378 image series acquired with 13 scanners from 4 manufacturers across 8 institutions using a harmonized protocol as well as several acquisition doses. Additionally, we present a methodology, baseline results and open-source code to assess image- and feature-level stability and liver tissue classification, promoting the development of AI harmonization strategies.
Clinnova Federated Learning Proof of Concept: Key Takeaways from a Cross-border Collaboration
Oct 03, 2024



Abstract:Clinnova, a collaborative initiative involving France, Germany, Switzerland, and Luxembourg, is dedicated to unlocking the power of precision medicine through data federation, standardization, and interoperability. This European Greater Region initiative seeks to create an interoperable European standard using artificial intelligence (AI) and data science to enhance healthcare outcomes and efficiency. Key components include multidisciplinary research centers, a federated biobanking strategy, a digital health innovation platform, and a federated AI strategy. It targets inflammatory bowel disease, rheumatoid diseases, and multiple sclerosis (MS), emphasizing data quality to develop AI algorithms for personalized treatment and translational research. The IHU Strasbourg (Institute of Minimal-invasive Surgery) has the lead in this initiative to develop the federated learning (FL) proof of concept (POC) that will serve as a foundation for advancing AI in healthcare. At its core, Clinnova-MS aims to enhance MS patient care by using FL to develop more accurate models that detect disease progression, guide interventions, and validate digital biomarkers across multiple sites. This technical report presents insights and key takeaways from the first cross-border federated POC on MS segmentation of MRI images within the Clinnova framework. While our work marks a significant milestone in advancing MS segmentation through cross-border collaboration, it also underscores the importance of addressing technical, logistical, and ethical considerations to realize the full potential of FL in healthcare settings.
Multi-centric AI Model for Unruptured Intracranial Aneurysm Detection and Volumetric Segmentation in 3D TOF-MRI
Aug 30, 2024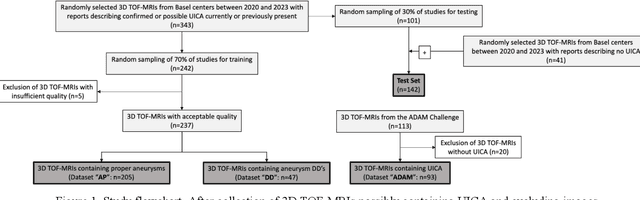
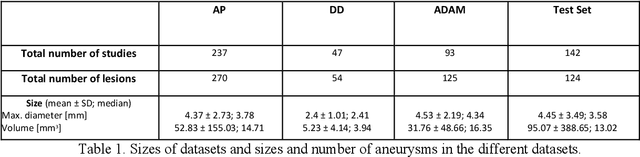
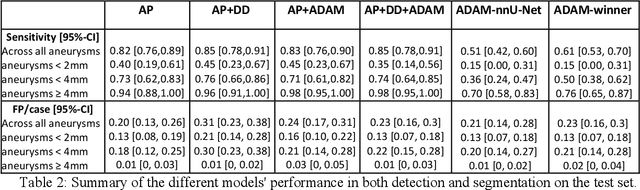
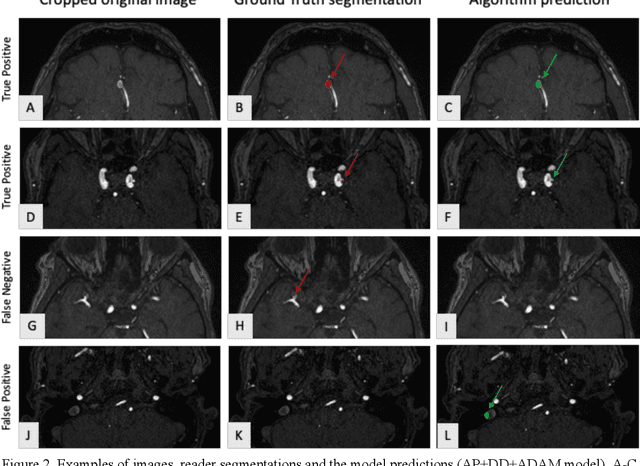
Abstract:Purpose: To develop an open-source nnU-Net-based AI model for combined detection and segmentation of unruptured intracranial aneurysms (UICA) in 3D TOF-MRI, and compare models trained on datasets with aneurysm-like differential diagnoses. Methods: This retrospective study (2020-2023) included 385 anonymized 3D TOF-MRI images from 364 patients (mean age 59 years, 60% female) at multiple centers plus 113 subjects from the ADAM challenge. Images featured untreated or possible UICAs and differential diagnoses. Four distinct training datasets were created, and the nnU-Net framework was used for model development. Performance was assessed on a separate test set using sensitivity and False Positive (FP)/case rate for detection, and DICE score and NSD (Normalized Surface Distance) with a 0.5mm threshold for segmentation. Statistical analysis included chi-square, Mann-Whitney-U, and Kruskal-Wallis tests, with significance set at p < 0.05. Results: Models achieved overall sensitivity between 82% and 85% and a FP/case rate of 0.20 to 0.31, with no significant differences (p = 0.90 and p = 0.16). The primary model showed 85% sensitivity and 0.23 FP/case rate, outperforming the ADAM-challenge winner (61%) and a nnU-Net trained on ADAM data (51%) in sensitivity (p < 0.05). It achieved a mean DICE score of 0.73 and an NSD of 0.84 for correctly detected UICA. Conclusions: Our open-source, nnU-Net-based AI model (available at 10.5281/zenodo.13386859) demonstrates high sensitivity, low false positive rates, and consistent segmentation accuracy for UICA detection and segmentation in 3D TOF-MRI, suggesting its potential to improve clinical diagnosis and for monitoring of UICA.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge