Md. Jahin Alam
An Optimized YOLOv5 Based Approach For Real-time Vehicle Detection At Road Intersections Using Fisheye Cameras
Feb 06, 2025



Abstract:Real time vehicle detection is a challenging task for urban traffic surveillance. Increase in urbanization leads to increase in accidents and traffic congestion in junction areas resulting in delayed travel time. In order to solve these problems, an intelligent system utilizing automatic detection and tracking system is significant. But this becomes a challenging task at road intersection areas which require a wide range of field view. For this reason, fish eye cameras are widely used in real time vehicle detection purpose to provide large area coverage and 360 degree view at junctions. However, it introduces challenges such as light glare from vehicles and street lights, shadow, non-linear distortion, scaling issues of vehicles and proper localization of small vehicles. To overcome each of these challenges, a modified YOLOv5 object detection scheme is proposed. YOLOv5 is a deep learning oriented convolutional neural network (CNN) based object detection method. The proposed scheme for detecting vehicles in fish-eye images consists of a light-weight day-night CNN classifier so that two different solutions can be implemented to address the day-night detection issues. Furthurmore, challenging instances are upsampled in the dataset for proper localization of vehicles and later on the detection model is ensembled and trained in different combination of vehicle datasets for better generalization, detection and accuracy. For testing, a real world fisheye dataset provided by the Video and Image Processing (VIP) Cup organizer ISSD has been used which includes images from video clips of different fisheye cameras at junction of different cities during day and night time. Experimental results show that our proposed model has outperformed the YOLOv5 model on the dataset by 13.7% mAP @ 0.5.
A Constrained Optimization approach for Ultrasound Shear Wave Speed Estimation with Time-Lateral Plane Cleaning
Jul 30, 2024



Abstract:Ultrasound shear wave elastography (SWE) is a noninvasive way to measure stiffness of soft tissue for medical diagnosis. In SWE imaging, an acoustic radiation force induces tissue displacement, which creates shear waves (SWs) that travel laterally through the medium. Finding the lateral arrival times of SWs at different tissue locations helps figure out the shear wave speed (SWS), which is directly linked to the stiffness of the medium. Traditional SWS estimation techniques, however, are not noise resilient enough handling noise and reflection artifacts filled data. This paper proposes new techniques to estimate SWS in both time and frequency domains. These new methods optimize a loss function that is based on the lateral signal shift parameter between known locations and is constrained by neighborhood displacement group shift determined from the time-lateral plane-denoised SW propagation data. The proposed constrained optimization is formed by coupling losses of local particles with a Gaussian kernel giving an optimum arrival time for the center particle by enforcing stiffness homogeneity in a small neighborhood to enable inherent noise resilience. The denoising scheme involves isolating the transitioning SW profile in each time-lateral plane, creating a parameterized mask. Moreover, lateral interpolation is performed to enhance reconstruction resolution and obtain increased displacement groups to enhance the reliability of the optimization. The proposed noise robust scheme is tested on a simulation and three experimental datasets. The performance of the method is compared with 3 ToF and 2 frequency-domain methods. The evaluations show visually and quantitatively superior and noise-robust reconstructions compared to state-of-the-art methods. Due to its high contrast and minimal error, the proposed technique can find its application in tissue health inspection and cancer diagnosis.
Robust CNN Multi-Nested-LSTM Framework with Compound Loss for Patch-based Multi-Push Ultrasound Shear Wave Imaging and Segmentation
Jul 30, 2024


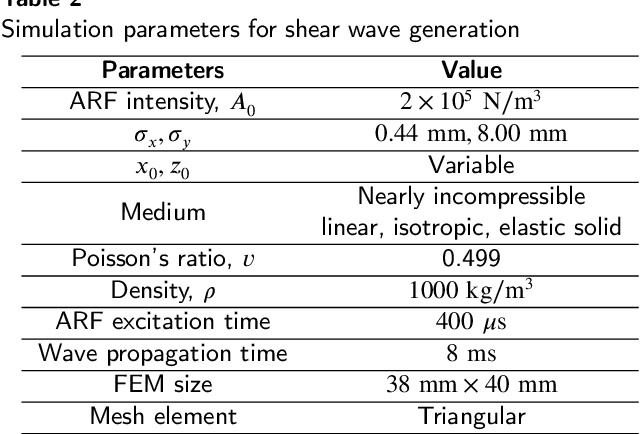
Abstract:Ultrasound Shear Wave Elastography (SWE) is a noteworthy tool for in-vivo noninvasive tissue pathology assessment. State-of-the-art techniques can generate reasonable estimates of tissue elasticity, but high-quality and noise-resiliency in SWE reconstruction have yet to demonstrate advancements. In this work, we propose a two-stage DL pipeline producing reliable reconstructions and denoise said reconstructions to obtain lower noise prevailing elasticity mappings. The reconstruction network consists of a Resnet3D Encoder to extract temporal context from the sequential multi-push data. The encoded features are sent to multiple Nested CNN LSTMs which process them in a temporal attention-guided windowing basis and map the 3D features to 2D using FFT-attention, which are then decoded into an elasticity map as primary reconstruction. The 2D maps from each multi-push region are merged and cleaned using a dual-decoder denoiser network, which independently denoises foreground and background before fusion. The post-denoiser generates a higher-quality reconstruction and an inclusion-segmentation mask. A multi-objective loss is designed to accommodate the denoising, fusing, and segmentation processes. The method is validated on sequential multi-push SWE motion data with multiple overlapping regions. A patch-based training procedure is introduced with network modifications to handle data scarcity. Evaluations produce 32.66dB PSNR, 43.19dB CNR in noisy simulation, and 22.44dB PSNR, 36.88dB CNR in experimental data, across all test samples. Moreover, IoUs (0.909 and 0.781) were quite satisfactory in the datasets. After comparing with other reported deep-learning approaches, our method proves quantitatively and qualitatively superior in dealing with noise influences in SWE data. From a performance point of view, our deep-learning pipeline has the potential to become utilitarian in the clinical domain.
CovTANet: A Hybrid Tri-level Attention Based Network for Lesion Segmentation, Diagnosis, and Severity Prediction of COVID-19 Chest CT Scans
Jan 03, 2021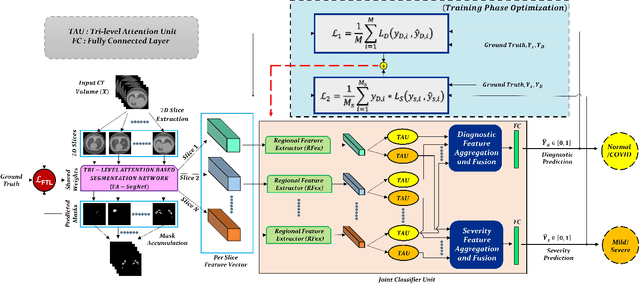
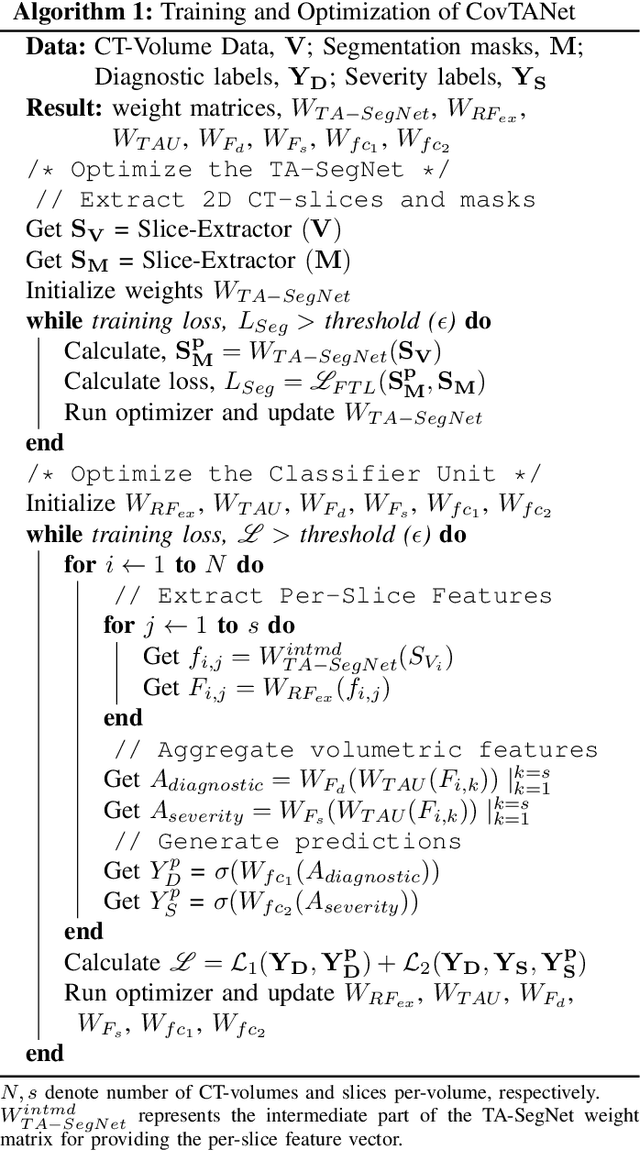
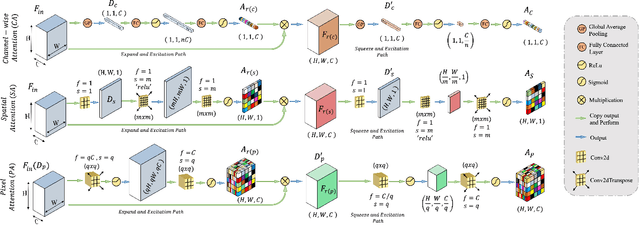
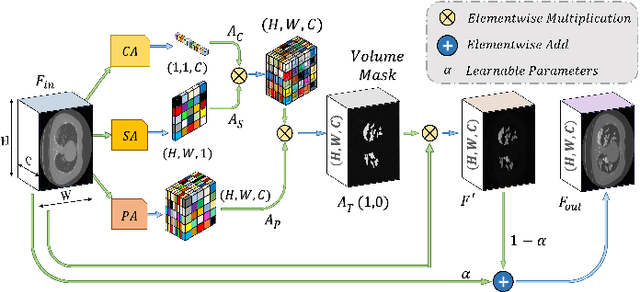
Abstract:Rapid and precise diagnosis of COVID-19 is one of the major challenges faced by the global community to control the spread of this overgrowing pandemic. In this paper, a hybrid neural network is proposed, named CovTANet, to provide an end-to-end clinical diagnostic tool for early diagnosis, lesion segmentation, and severity prediction of COVID-19 utilizing chest computer tomography (CT) scans. A multi-phase optimization strategy is introduced for solving the challenges of complicated diagnosis at a very early stage of infection, where an efficient lesion segmentation network is optimized initially which is later integrated into a joint optimization framework for the diagnosis and severity prediction tasks providing feature enhancement of the infected regions. Moreover, for overcoming the challenges with diffused, blurred, and varying shaped edges of COVID lesions with novel and diverse characteristics, a novel segmentation network is introduced, namely Tri-level Attention-based Segmentation Network (TA-SegNet). This network has significantly reduced semantic gaps in subsequent encoding decoding stages, with immense parallelization of multi-scale features for faster convergence providing considerable performance improvement over traditional networks. Furthermore, a novel tri-level attention mechanism has been introduced, which is repeatedly utilized over the network, combining channel, spatial, and pixel attention schemes for faster and efficient generalization of contextual information embedded in the feature map through feature re-calibration and enhancement operations. Outstanding performances have been achieved in all three-tasks through extensive experimentation on a large publicly available dataset containing 1110 chest CT-volumes that signifies the effectiveness of the proposed scheme at the current stage of the pandemic.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge