Max Dünnwald
Automated SSIM Regression for Detection and Quantification of Motion Artefacts in Brain MR Images
Jun 14, 2022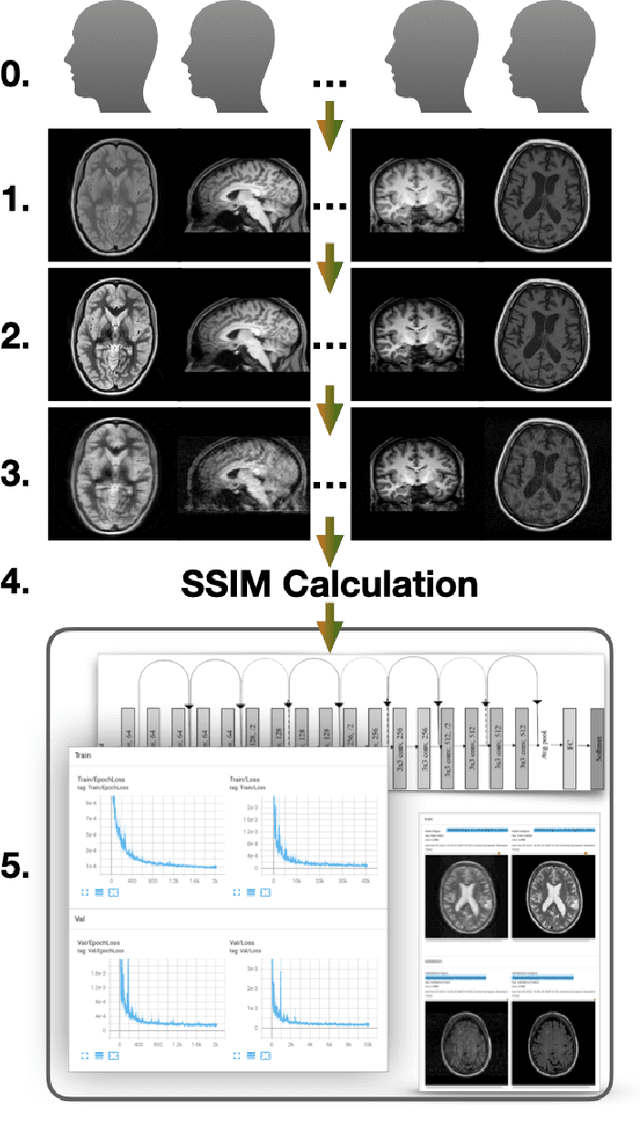
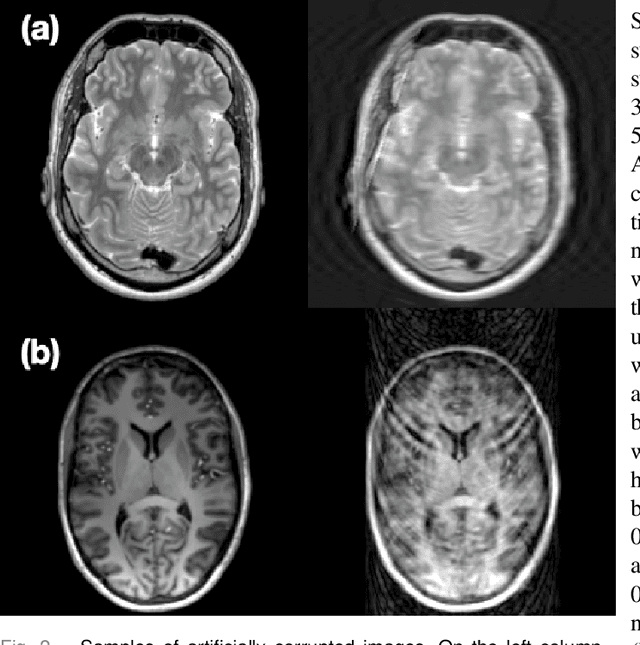
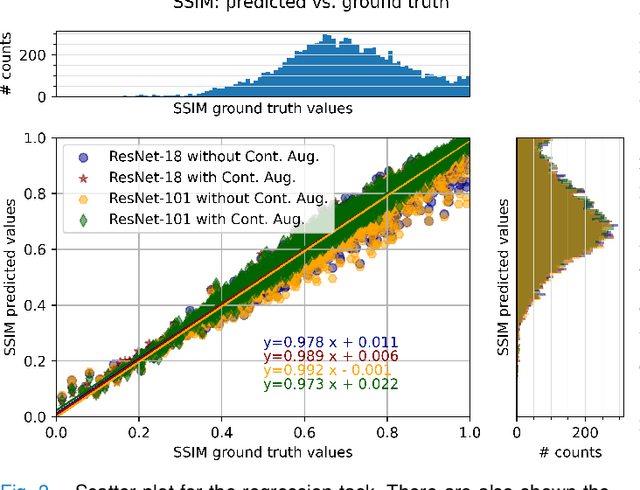
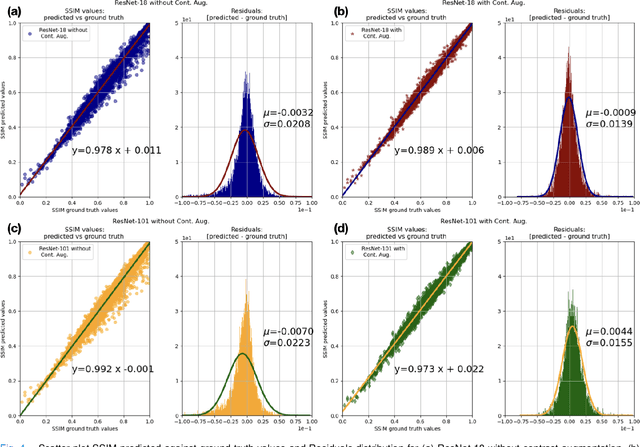
Abstract:Motion artefacts in magnetic resonance brain images are a crucial issue. The assessment of MR image quality is fundamental before proceeding with the clinical diagnosis. If the motion artefacts alter a correct delineation of structure and substructures of the brain, lesions, tumours and so on, the patients need to be re-scanned. Otherwise, neuro-radiologists could report an inaccurate or incorrect diagnosis. The first step right after scanning a patient is the "\textit{image quality assessment}" in order to decide if the acquired images are diagnostically acceptable. An automated image quality assessment based on the structural similarity index (SSIM) regression through a residual neural network has been proposed here, with the possibility to perform also the classification in different groups - by subdividing with SSIM ranges. This method predicts SSIM values of an input image in the absence of a reference ground truth image. The networks were able to detect motion artefacts, and the best performance for the regression and classification task has always been achieved with ResNet-18 with contrast augmentation. Mean and standard deviation of residuals' distribution were $\mu=-0.0009$ and $\sigma=0.0139$, respectively. Whilst for the classification task in 3, 5 and 10 classes, the best accuracies were 97, 95 and 89\%, respectively. The obtained results show that the proposed method could be a tool in supporting neuro-radiologists and radiographers in evaluating the image quality before the diagnosis.
StRegA: Unsupervised Anomaly Detection in Brain MRIs using a Compact Context-encoding Variational Autoencoder
Jan 31, 2022



Abstract:Expert interpretation of anatomical images of the human brain is the central part of neuro-radiology. Several machine learning-based techniques have been proposed to assist in the analysis process. However, the ML models typically need to be trained to perform a specific task, e.g., brain tumour segmentation or classification. Not only do the corresponding training data require laborious manual annotations, but a wide variety of abnormalities can be present in a human brain MRI - even more than one simultaneously, which renders representation of all possible anomalies very challenging. Hence, a possible solution is an unsupervised anomaly detection (UAD) system that can learn a data distribution from an unlabelled dataset of healthy subjects and then be applied to detect out of distribution samples. Such a technique can then be used to detect anomalies - lesions or abnormalities, for example, brain tumours, without explicitly training the model for that specific pathology. Several Variational Autoencoder (VAE) based techniques have been proposed in the past for this task. Even though they perform very well on controlled artificially simulated anomalies, many of them perform poorly while detecting anomalies in clinical data. This research proposes a compact version of the "context-encoding" VAE (ceVAE) model, combined with pre and post-processing steps, creating a UAD pipeline (StRegA), which is more robust on clinical data, and shows its applicability in detecting anomalies such as tumours in brain MRIs. The proposed pipeline achieved a Dice score of 0.642$\pm$0.101 while detecting tumours in T2w images of the BraTS dataset and 0.859$\pm$0.112 while detecting artificially induced anomalies, while the best performing baseline achieved 0.522$\pm$0.135 and 0.783$\pm$0.111, respectively.
ShuffleUNet: Super resolution of diffusion-weighted MRIs using deep learning
Feb 25, 2021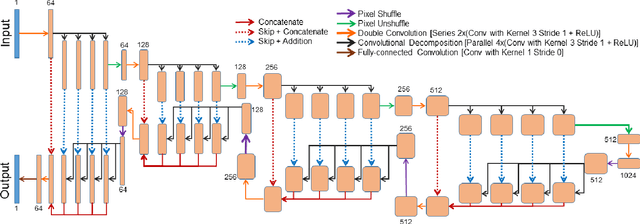
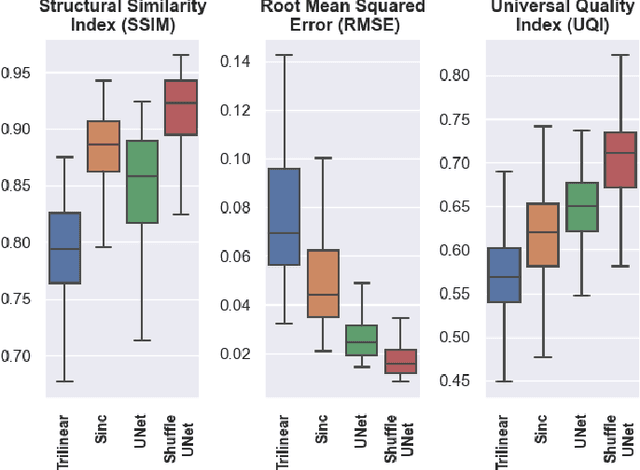
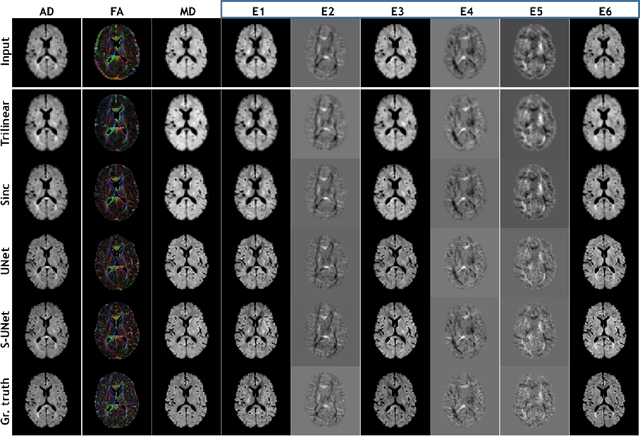
Abstract:Diffusion-weighted magnetic resonance imaging (DW-MRI) can be used to characterise the microstructure of the nervous tissue, e.g. to delineate brain white matter connections in a non-invasive manner via fibre tracking. Magnetic Resonance Imaging (MRI) in high spatial resolution would play an important role in visualising such fibre tracts in a superior manner. However, obtaining an image of such resolution comes at the expense of longer scan time. Longer scan time can be associated with the increase of motion artefacts, due to the patient's psychological and physical conditions. Single Image Super-Resolution (SISR), a technique aimed to obtain high-resolution (HR) details from one single low-resolution (LR) input image, achieved with Deep Learning, is the focus of this study. Compared to interpolation techniques or sparse-coding algorithms, deep learning extracts prior knowledge from big datasets and produces superior MRI images from the low-resolution counterparts. In this research, a deep learning based super-resolution technique is proposed and has been applied for DW-MRI. Images from the IXI dataset have been used as the ground-truth and were artificially downsampled to simulate the low-resolution images. The proposed method has shown statistically significant improvement over the baselines and achieved an SSIM of $0.913\pm0.045$.
Retrospective Motion Correction of MR Images using Prior-Assisted Deep Learning
Nov 28, 2020

Abstract:In MRI, motion artefacts are among the most common types of artefacts. They can degrade images and render them unusable for accurate diagnosis. Traditional methods, such as prospective or retrospective motion correction, have been proposed to avoid or alleviate motion artefacts. Recently, several other methods based on deep learning approaches have been proposed to solve this problem. This work proposes to enhance the performance of existing deep learning models by the inclusion of additional information present as image priors. The proposed approach has shown promising results and will be further investigated for clinical validity.
Spinal Metastases Segmentation in MR Imaging using Deep Convolutional Neural Networks
Jan 28, 2020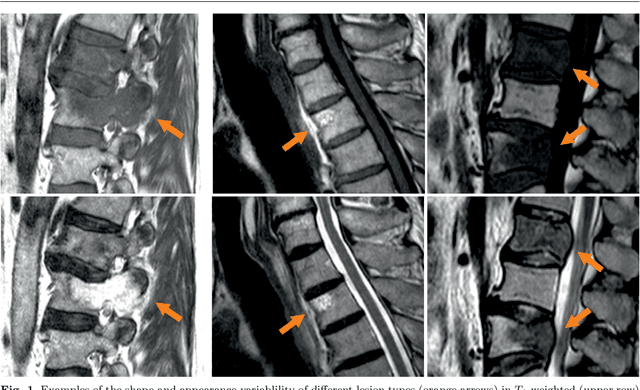
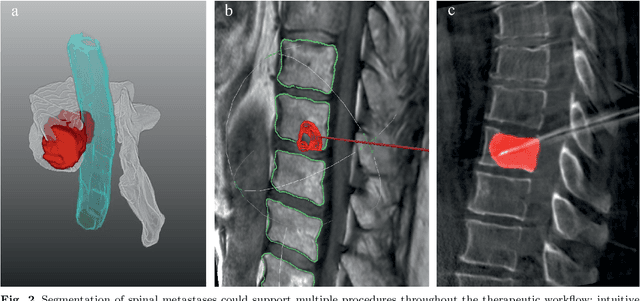
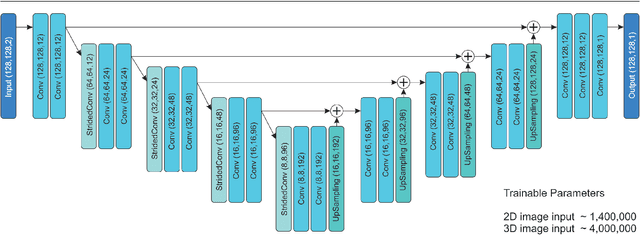
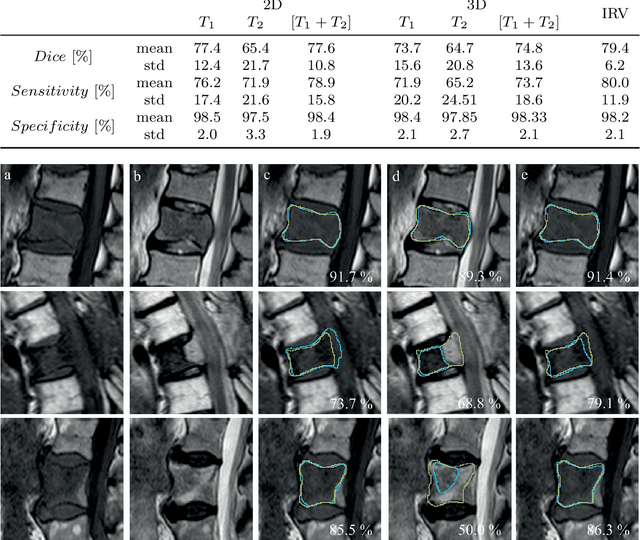
Abstract:This study's objective was to segment spinal metastases in diagnostic MR images using a deep learning-based approach. Segmentation of such lesions can present a pivotal step towards enhanced therapy planning and validation, as well as intervention support during minimally invasive and image-guided surgeries like radiofrequency ablations. For this purpose, we used a U-Net like architecture trained with 40 clinical cases including both, lytic and sclerotic lesion types and various MR sequences. Our proposed method was evaluated with regards to various factors influencing the segmentation quality, e.g. the used MR sequences and the input dimension. We quantitatively assessed our experiments using Dice coefficients, sensitivity and specificity rates. Compared to expertly annotated lesion segmentations, the experiments yielded promising results with average Dice scores up to 77.6% and mean sensitivity rates up to 78.9%. To our best knowledge, our proposed study is one of the first to tackle this particular issue, which limits direct comparability with related works. In respect to similar deep learning-based lesion segmentations, e.g. in liver MR images or spinal CT images, our experiments showed similar or in some respects superior segmentation quality. Overall, our automatic approach can provide almost expert-like segmentation accuracy in this challenging and ambitious task.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge