Matthew Perez
Beyond Binary: Multiclass Paraphasia Detection with Generative Pretrained Transformers and End-to-End Models
Jul 16, 2024
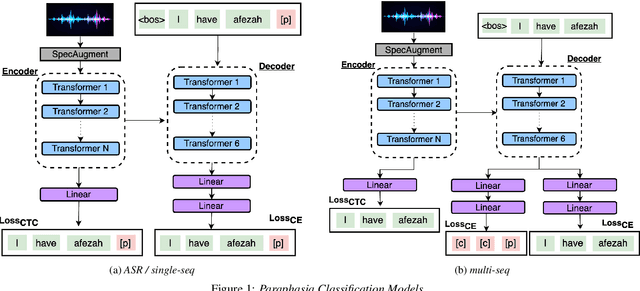
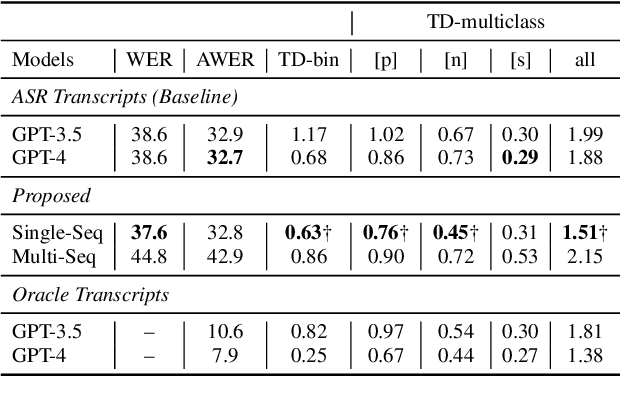
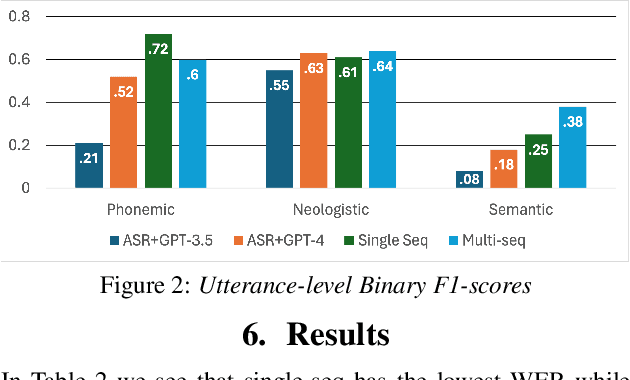
Abstract:Aphasia is a language disorder that can lead to speech errors known as paraphasias, which involve the misuse, substitution, or invention of words. Automatic paraphasia detection can help those with Aphasia by facilitating clinical assessment and treatment planning options. However, most automatic paraphasia detection works have focused solely on binary detection, which involves recognizing only the presence or absence of a paraphasia. Multiclass paraphasia detection represents an unexplored area of research that focuses on identifying multiple types of paraphasias and where they occur in a given speech segment. We present novel approaches that use a generative pretrained transformer (GPT) to identify paraphasias from transcripts as well as two end-to-end approaches that focus on modeling both automatic speech recognition (ASR) and paraphasia classification as multiple sequences vs. a single sequence. We demonstrate that a single sequence model outperforms GPT baselines for multiclass paraphasia detection.
Seq2seq for Automatic Paraphasia Detection in Aphasic Speech
Dec 16, 2023Abstract:Paraphasias are speech errors that are often characteristic of aphasia and they represent an important signal in assessing disease severity and subtype. Traditionally, clinicians manually identify paraphasias by transcribing and analyzing speech-language samples, which can be a time-consuming and burdensome process. Identifying paraphasias automatically can greatly help clinicians with the transcription process and ultimately facilitate more efficient and consistent aphasia assessment. Previous research has demonstrated the feasibility of automatic paraphasia detection by training an automatic speech recognition (ASR) model to extract transcripts and then training a separate paraphasia detection model on a set of hand-engineered features. In this paper, we propose a novel, sequence-to-sequence (seq2seq) model that is trained end-to-end (E2E) to perform both ASR and paraphasia detection tasks. We show that the proposed model outperforms the previous state-of-the-art approach for both word-level and utterance-level paraphasia detection tasks and provide additional follow-up evaluations to further understand the proposed model behavior.
Articulatory Coordination for Speech Motor Tracking in Huntington Disease
Sep 28, 2021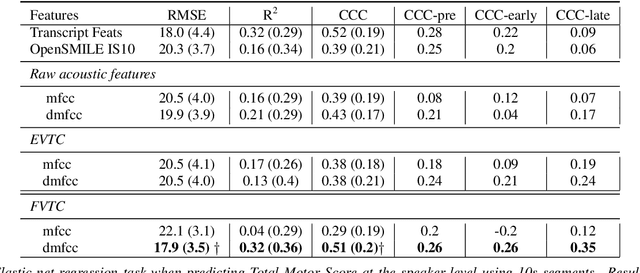
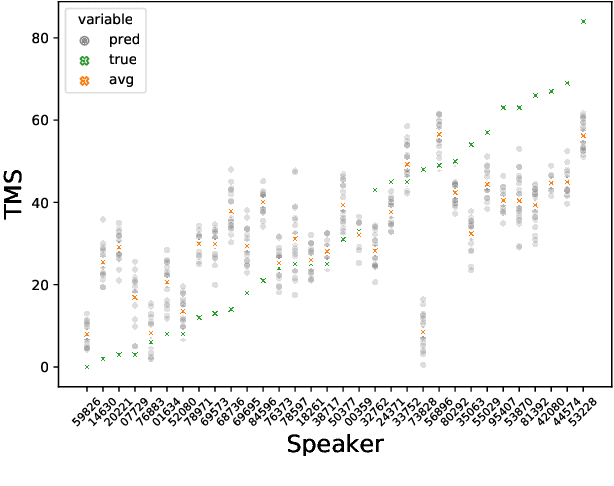
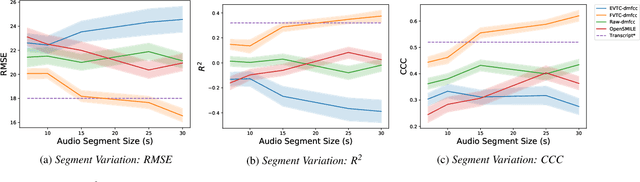
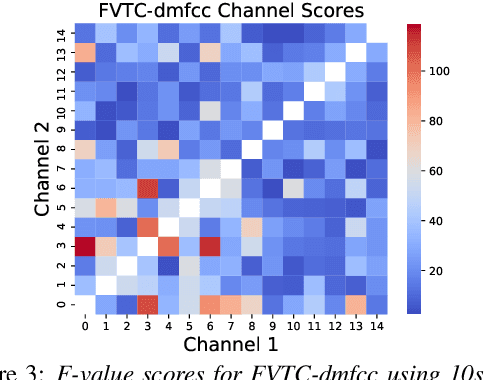
Abstract:Huntington Disease (HD) is a progressive disorder which often manifests in motor impairment. Motor severity (captured via motor score) is a key component in assessing overall HD severity. However, motor score evaluation involves in-clinic visits with a trained medical professional, which are expensive and not always accessible. Speech analysis provides an attractive avenue for tracking HD severity because speech is easy to collect remotely and provides insight into motor changes. HD speech is typically characterized as having irregular articulation. With this in mind, acoustic features that can capture vocal tract movement and articulatory coordination are particularly promising for characterizing motor symptom progression in HD. In this paper, we present an experiment that uses Vocal Tract Coordination (VTC) features extracted from read speech to estimate a motor score. When using an elastic-net regression model, we find that VTC features significantly outperform other acoustic features across varied-length audio segments, which highlights the effectiveness of these features for both short- and long-form reading tasks. Lastly, we analyze the F-value scores of VTC features to visualize which channels are most related to motor score. This work enables future research efforts to consider VTC features for acoustic analyses which target HD motor symptomatology tracking.
Classification of Huntington Disease using Acoustic and Lexical Features
Aug 07, 2020
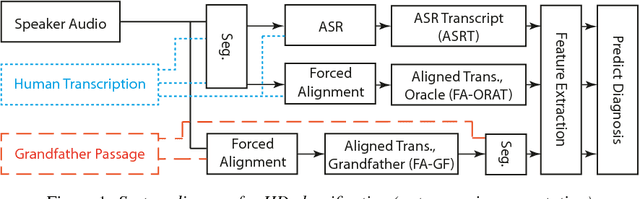

Abstract:Speech is a critical biomarker for Huntington Disease (HD), with changes in speech increasing in severity as the disease progresses. Speech analyses are currently conducted using either transcriptions created manually by trained professionals or using global rating scales. Manual transcription is both expensive and time-consuming and global rating scales may lack sufficient sensitivity and fidelity. Ultimately, what is needed is an unobtrusive measure that can cheaply and continuously track disease progression. We present first steps towards the development of such a system, demonstrating the ability to automatically differentiate between healthy controls and individuals with HD using speech cues. The results provide evidence that objective analyses can be used to support clinical diagnoses, moving towards the tracking of symptomatology outside of laboratory and clinical environments.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge