Kwangsik Nho
Alzheimer's Disease Diagnosis via Deep Factorization Machine Models
Aug 12, 2021


Abstract:The current state-of-the-art deep neural networks (DNNs) for Alzheimer's Disease diagnosis use different biomarker combinations to classify patients, but do not allow extracting knowledge about the interactions of biomarkers. However, to improve our understanding of the disease, it is paramount to extract such knowledge from the learned model. In this paper, we propose a Deep Factorization Machine model that combines the ability of DNNs to learn complex relationships and the ease of interpretability of a linear model. The proposed model has three parts: (i) an embedding layer to deal with sparse categorical data, (ii) a Factorization Machine to efficiently learn pairwise interactions, and (iii) a DNN to implicitly model higher order interactions. In our experiments on data from the Alzheimer's Disease Neuroimaging Initiative, we demonstrate that our proposed model classifies cognitive normal, mild cognitive impaired, and demented patients more accurately than competing models. In addition, we show that valuable knowledge about the interactions among biomarkers can be obtained.
Deep Learning in Alzheimer's disease: Diagnostic Classification and Prognostic Prediction using Neuroimaging Data
May 06, 2019
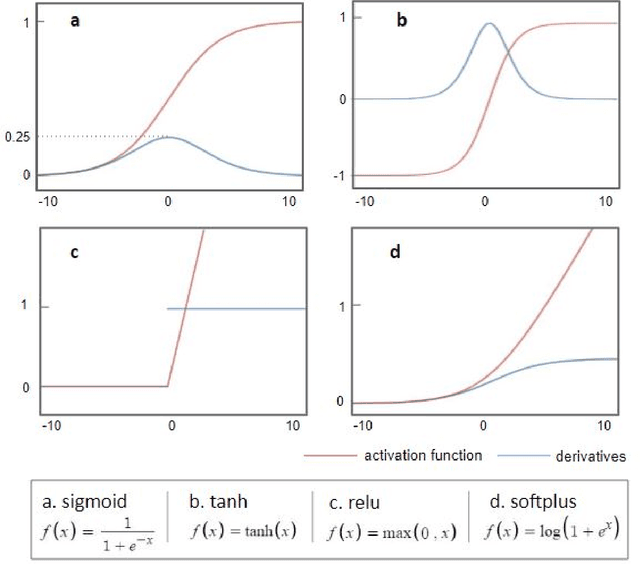
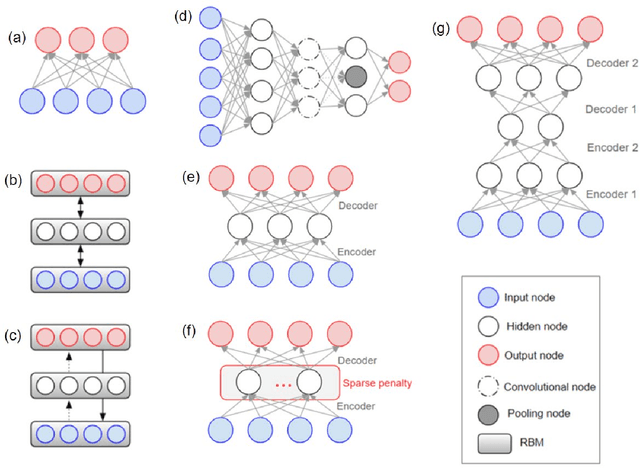
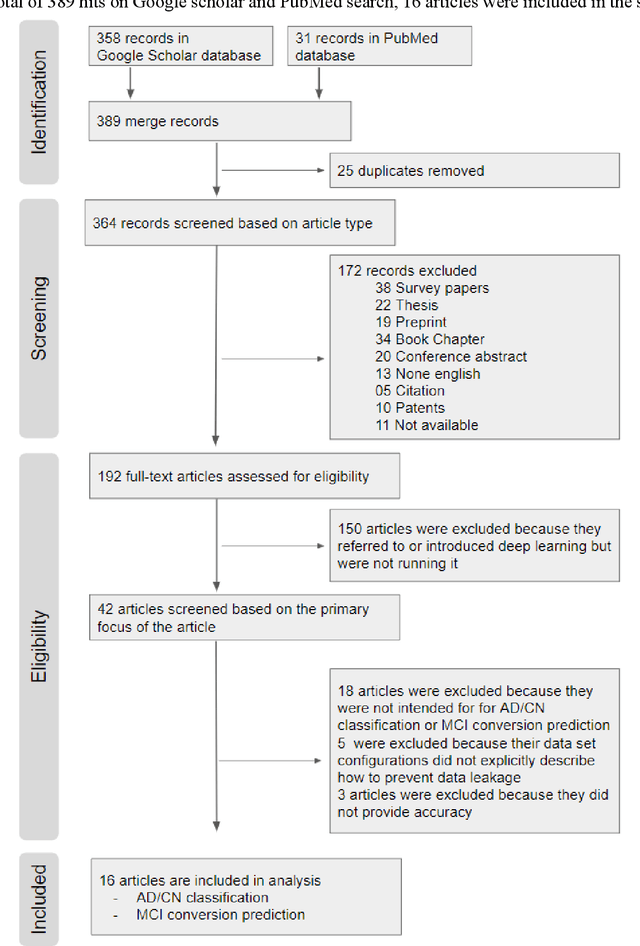
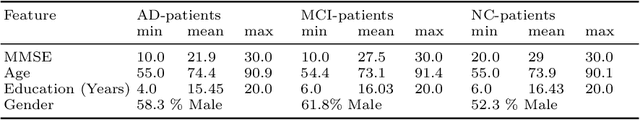
Abstract:The application of deep learning to early detection and automated classification of Alzheimer's disease (AD) has recently gained considerable attention as rapid progress in neuroimaging techniques has generated large-scale multimodal neuroimaging data. Here we systematically reviewed publications, where deep learning approaches and neuroimaging data were used for diagnostic classification of AD. A PubMed and google scholar search was performed to find deep learning papers for AD published between January 2013 and July 2018, which were reviewed, evaluated, and classified by algorithms and neuroimaging types, and findings were summarized. The diagnostic classification of AD using deep learning approaches and neuroimaging data was examined in 16 studies. The approach to combine traditional machine learning for classification and stacked auto-encoder (SAE) for feature selection has produced accuracies of up to 98.8% for AD classification and 83.7% for prediction of conversion from mild cognitive impairment (MCI), a prodromal stage of AD, to AD. Deep learning approaches such as convolutional neural network (CNN) or recurrent neural network (RNN) using neuroimaging data without preprocessing for feature selection have yielded accuracies of up to 96.0% for AD classification and 84.2% for MCI conversion prediction. Furthermore, the best classification performance was obtained when multimodal neuroimaging data as well as fluid biomarkers were integrated. Deep learning approaches without preprocessing neuroimaging data for feature selection, a major bottleneck of traditional machining learning in high-dimensional data, continue to improve their performance and to show great promise in the diagnostic classification of AD using multimodal neuroimaging data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge