Kent K. Yamamoto
The OncoReach Stylet for Brachytherapy: Design Evaluation and Pilot Study
Jan 20, 2026Abstract:Cervical cancer accounts for a significant portion of the global cancer burden among women. Interstitial brachytherapy (ISBT) is a standard procedure for treating cervical cancer; it involves placing a radioactive source through a straight hollow needle within or in close proximity to the tumor and surrounding tissue. However, the use of straight needles limits surgical planning to a linear needle path. We present the OncoReach stylet, a handheld, tendon-driven steerable stylet designed for compatibility with standard ISBT 15- and 13-gauge needles. Building upon our prior work, we evaluated design parameters like needle gauge, spherical joint count and spherical joint placement, including an asymmetric disk design to identify a configuration that maximizes bending compliance while retaining axial stiffness. Free space experiments quantified tip deflection across configurations, and a two-tube Cosserat rod model accurately predicted the centerline shape of the needle for most trials. The best performing configuration was integrated into a reusable handheld prototype that enables manual actuation. A patient-derived, multi-composite phantom model of the uterus and pelvis was developed to conduct a pilot study of the OncoReach steerable stylet with one expert user. Results showed the ability to steer from less-invasive, medial entry points to reach the lateral-most targets, underscoring the significance of steerable stylets.
Tendon-Actuated Concentric Tube Endonasal Robot (TACTER)
Apr 28, 2025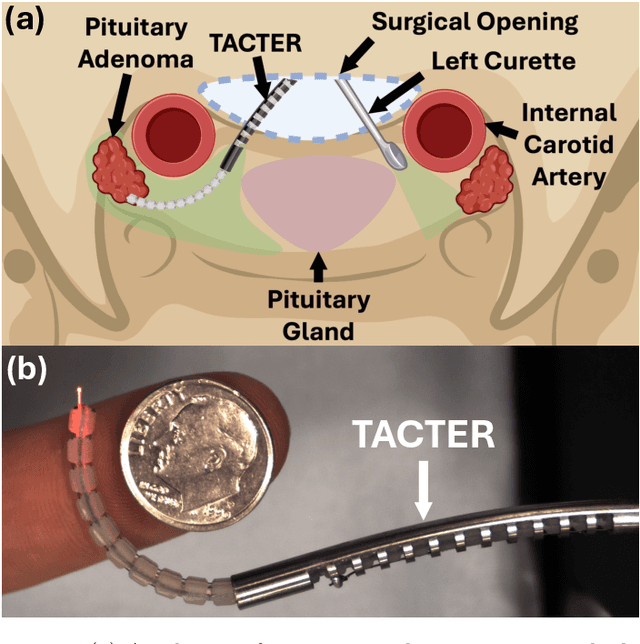
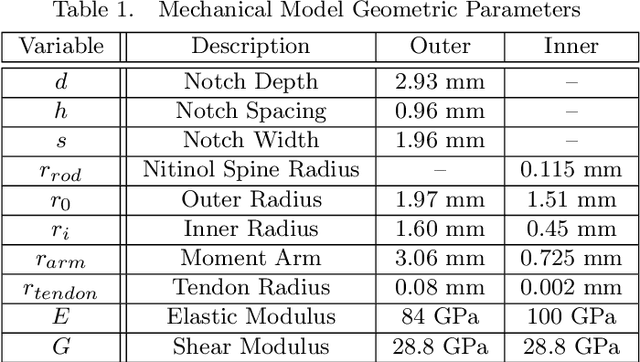
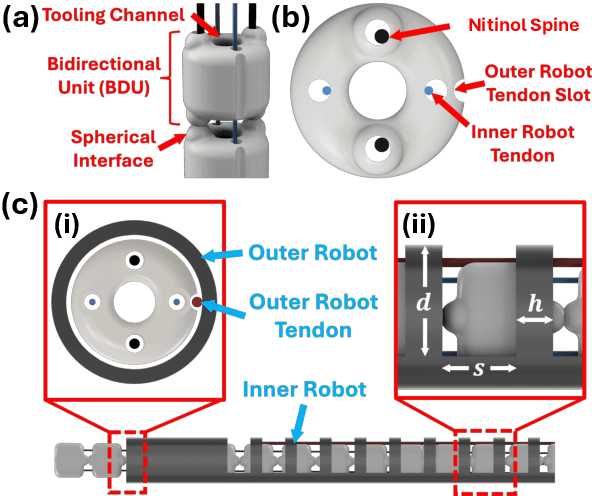
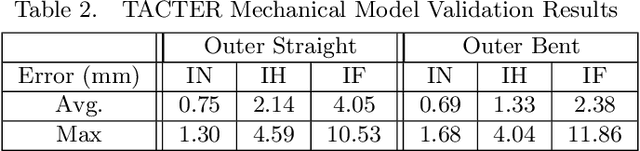
Abstract:Endoscopic endonasal approaches (EEA) have become more prevalent for minimally invasive skull base and sinus surgeries. However, rigid scopes and tools significantly decrease the surgeon's ability to operate in tight anatomical spaces and avoid critical structures such as the internal carotid artery and cranial nerves. This paper proposes a novel tendon-actuated concentric tube endonasal robot (TACTER) design in which two tendon-actuated robots are concentric to each other, resulting in an outer and inner robot that can bend independently. The outer robot is a unidirectionally asymmetric notch (UAN) nickel-titanium robot, and the inner robot is a 3D-printed bidirectional robot, with a nickel-titanium bending member. In addition, the inner robot can translate axially within the outer robot, allowing the tool to traverse through structures while bending, thereby executing follow-the-leader motion. A Cosserat-rod based mechanical model is proposed that uses tendon tension of both tendon-actuated robots and the relative translation between the robots as inputs and predicts the TACTER tip position for varying input parameters. The model is validated with experiments, and a human cadaver experiment is presented to demonstrate maneuverability from the nostril to the sphenoid sinus. This work presents the first tendon-actuated concentric tube (TACT) dexterous robotic tool capable of performing follow-the-leader motion within natural nasal orifices to cover workspaces typically required for a successful EEA.
Towards the Development of a Tendon-Actuated Galvanometer for Endoscopic Surgical Laser Scanning
Jun 05, 2024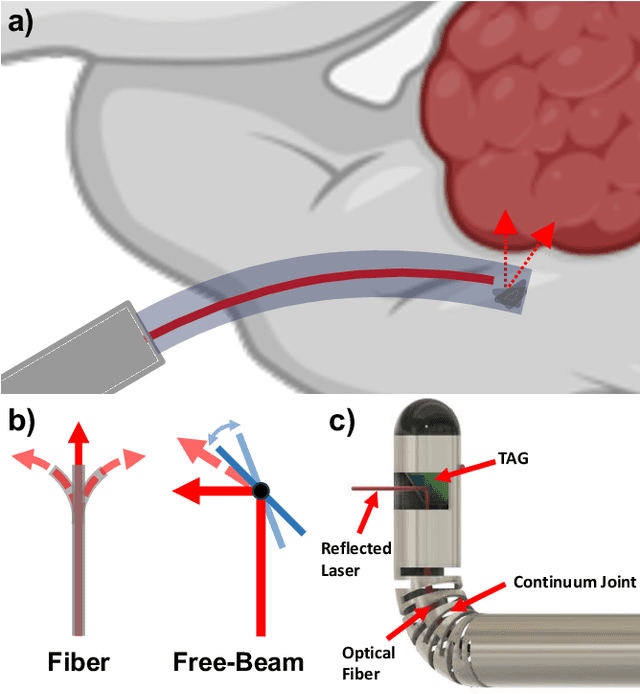
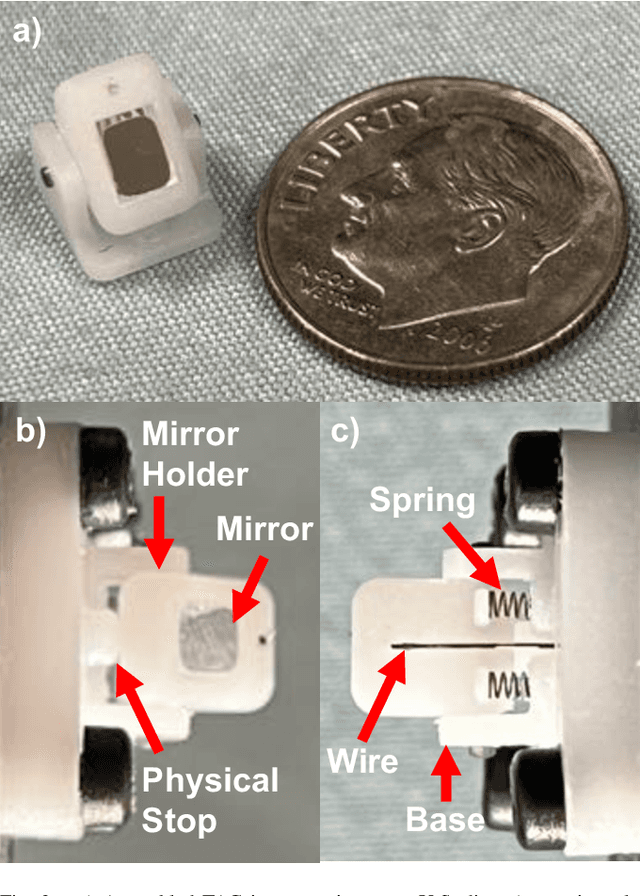
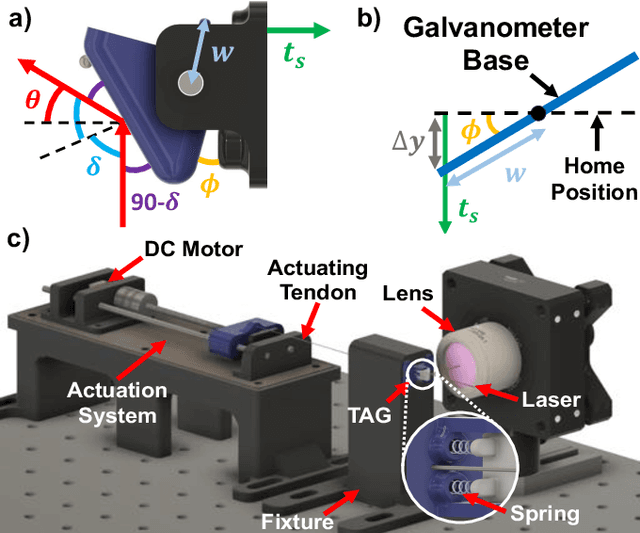
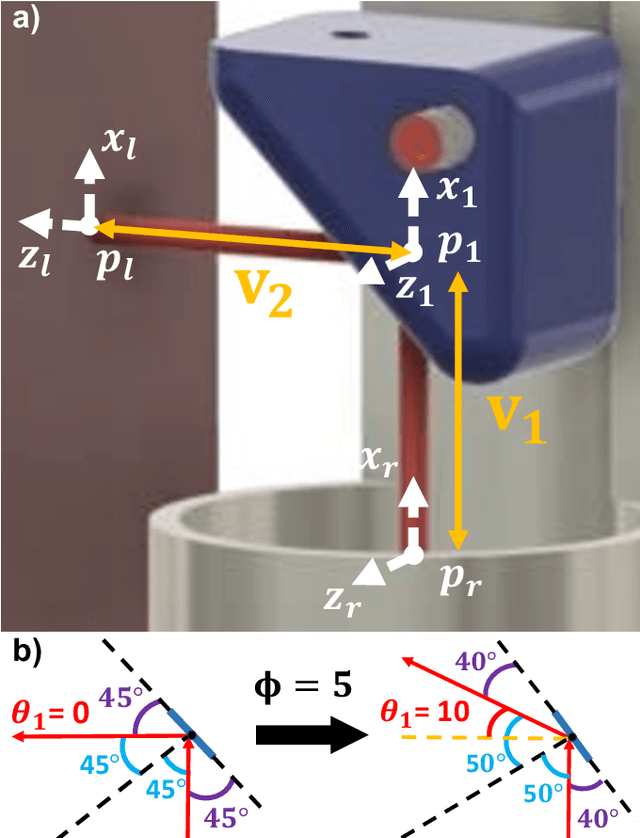
Abstract:There is a need for precision pathological sensing, imaging, and tissue manipulation in neurosurgical procedures, such as brain tumor resection. Precise tumor margin identification and resection can prevent further growth and protect critical structures. Surgical lasers with small laser diameters and steering capabilities can allow for new minimally invasive procedures by traversing through complex anatomy, then providing energy to sense, visualize, and affect tissue. In this paper, we present the design of a small-scale tendon-actuated galvanometer (TAG) that can serve as an end-effector tool for a steerable surgical laser. The galvanometer sensor design, fabrication, and kinematic modeling are presented and derived. It can accurately rotate up to 30.14 degrees (or a laser reflection angle of 60.28 degrees). A kinematic mapping of input tendon stroke to output galvanometer angle change and a forward-kinematics model relating the end of the continuum joint to the laser end-point are derived and validated.
An FBG-based Stiffness Estimation Sensor for In-vivo Diagnostics
May 30, 2024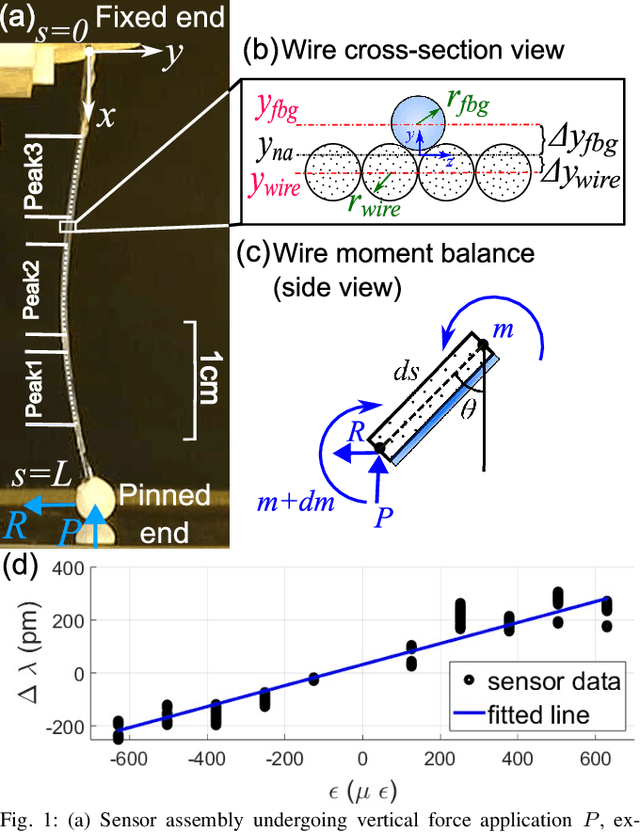
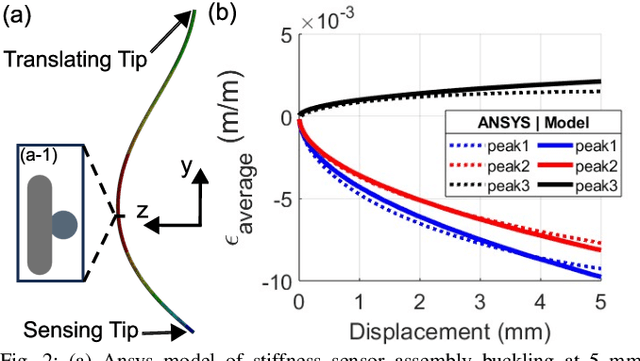
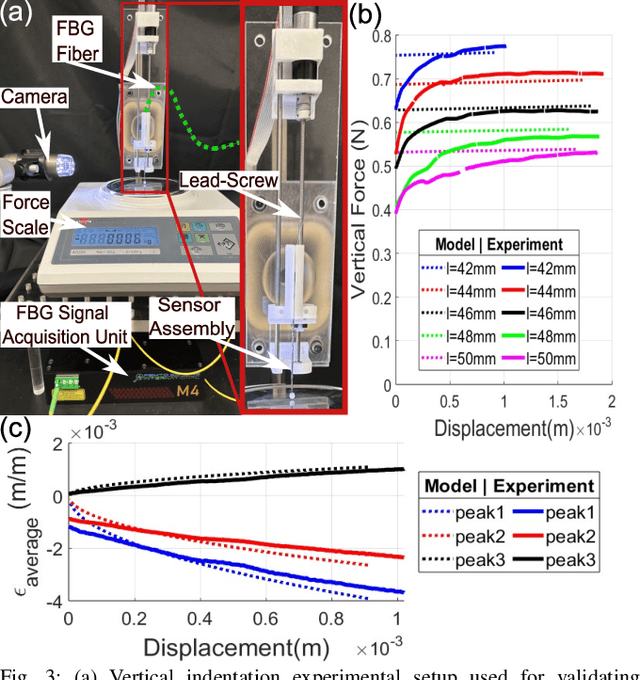
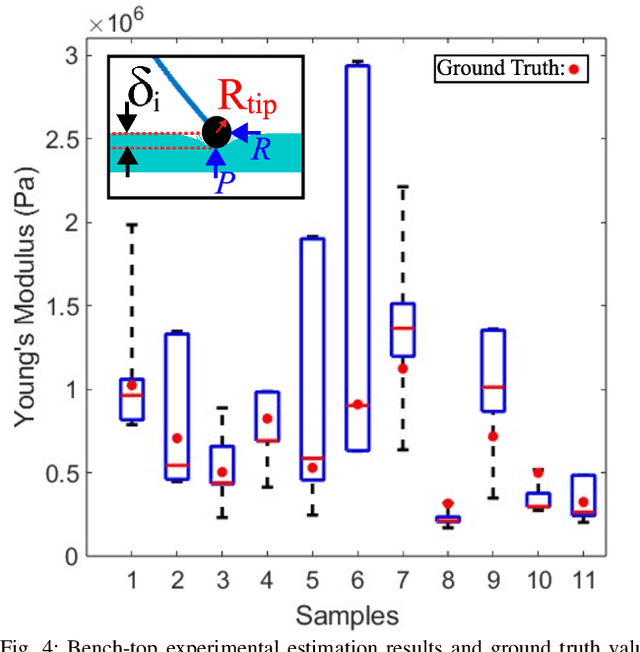
Abstract:In-vivo tissue stiffness identification can be useful in pulmonary fibrosis diagnostics and minimally invasive tumor identification, among many other applications. In this work, we propose a palpation-based method for tissue stiffness estimation that uses a sensorized beam buckled onto the surface of a tissue. Fiber Bragg Gratings (FBGs) are used in our sensor as a shape-estimation modality to get real-time beam shape, even while the device is not visually monitored. A mechanical model is developed to predict the behavior of a buckling beam and is validated using finite element analysis and bench-top testing with phantom tissue samples (made of PDMS and PA-Gel). Bench-top estimations were conducted and the results were compared with the actual stiffness values. Mean RMSE and standard deviation (from the actual stiffnesses) values of 413.86 KPa and 313.82 KPa were obtained. Estimations for softer samples were relatively closer to the actual values. Ultimately, we used the stiffness sensor within a mock concentric tube robot as a demonstration of \textit{in-vivo} sensor feasibility. Bench-top trials with and without the robot demonstrate the effectiveness of this unique sensing modality in \textit{in-vivo} applications.
Surface-Enhanced Raman Spectroscopy and Transfer Learning Toward Accurate Reconstruction of the Surgical Zone
Jan 16, 2024Abstract:Raman spectroscopy, a photonic modality based on the inelastic backscattering of coherent light, is a valuable asset to the intraoperative sensing space, offering non-ionizing potential and highly-specific molecular fingerprint-like spectroscopic signatures that can be used for diagnosis of pathological tissue in the dynamic surgical field. Though Raman suffers from weakness in intensity, Surface-Enhanced Raman Spectroscopy (SERS), which uses metal nanostructures to amplify Raman signals, can achieve detection sensitivities that rival traditional photonic modalities. In this study, we outline a robotic Raman system that can reliably pinpoint the location and boundaries of a tumor embedded in healthy tissue, modeled here as a tissue-mimicking phantom with selectively infused Gold Nanostar regions. Further, due to the relative dearth of collected biological SERS or Raman data, we implement transfer learning to achieve 100% validation classification accuracy for Gold Nanostars compared to Control Agarose, thus providing a proof-of-concept for Raman-based deep learning training pipelines. We reconstruct a surgical field of 30x60mm in 10.2 minutes, and achieve 98.2% accuracy, preserving relative measurements between features in the phantom. We also achieve an 84.3% Intersection-over-Union score, which is the extent of overlap between the ground truth and predicted reconstructions. Lastly, we also demonstrate that the Raman system and classification algorithm do not discern based on sample color, but instead on presence of SERS agents. This study provides a crucial step in the translation of intelligent Raman systems in intraoperative oncological spaces.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge