Kannie W. Y. Chan
pyMEAL: A Multi-Encoder Augmentation-Aware Learning for Robust and Generalizable Medical Image Translation
May 30, 2025Abstract:Medical imaging is critical for diagnostics, but clinical adoption of advanced AI-driven imaging faces challenges due to patient variability, image artifacts, and limited model generalization. While deep learning has transformed image analysis, 3D medical imaging still suffers from data scarcity and inconsistencies due to acquisition protocols, scanner differences, and patient motion. Traditional augmentation uses a single pipeline for all transformations, disregarding the unique traits of each augmentation and struggling with large data volumes. To address these challenges, we propose a Multi-encoder Augmentation-Aware Learning (MEAL) framework that leverages four distinct augmentation variants processed through dedicated encoders. Three fusion strategies such as concatenation (CC), fusion layer (FL), and adaptive controller block (BD) are integrated to build multi-encoder models that combine augmentation-specific features before decoding. MEAL-BD uniquely preserves augmentation-aware representations, enabling robust, protocol-invariant feature learning. As demonstrated in a Computed Tomography (CT)-to-T1-weighted Magnetic Resonance Imaging (MRI) translation study, MEAL-BD consistently achieved the best performance on both unseen- and predefined-test data. On both geometric transformations (like rotations and flips) and non-augmented inputs, MEAL-BD outperformed other competing methods, achieving higher mean peak signal-to-noise ratio (PSNR) and structural similarity index measure (SSIM) scores. These results establish MEAL as a reliable framework for preserving structural fidelity and generalizing across clinically relevant variability. By reframing augmentation as a source of diverse, generalizable features, MEAL supports robust, protocol-invariant learning, advancing clinically reliable medical imaging solutions.
Implicit Regression in Subspace for High-Sensitivity CEST Imaging
Jul 09, 2024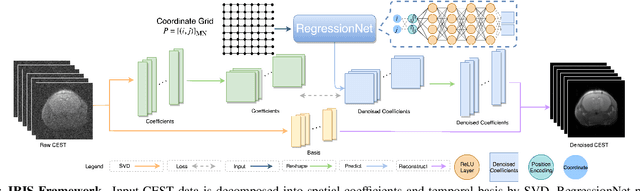
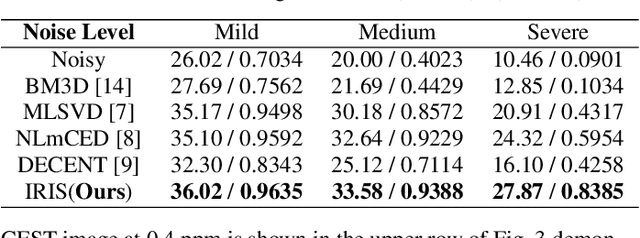
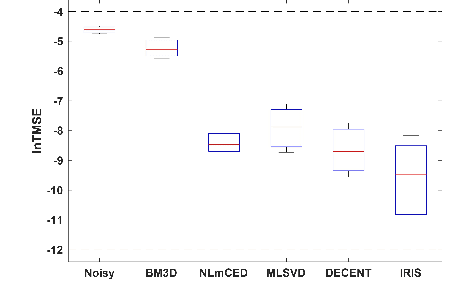
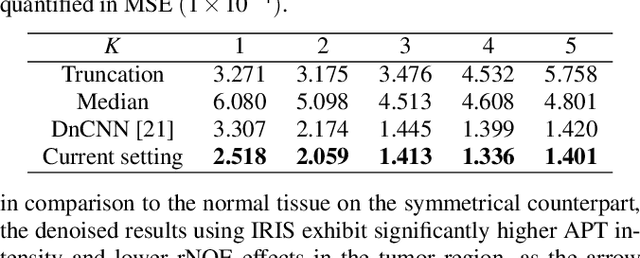
Abstract:Chemical Exchange Saturation Transfer (CEST) MRI demonstrates its capability in significantly enhancing the detection of proteins and metabolites with low concentrations through exchangeable protons. The clinical application of CEST, however, is constrained by its low contrast and low signal-to-noise ratio (SNR) in the acquired data. Denoising, as one of the post-processing stages for CEST data, can effectively improve the accuracy of CEST quantification. In this work, by modeling spatial variant z-spectrums into low-dimensional subspace, we introduce Implicit Regression in Subspace (IRIS), which is an unsupervised denoising algorithm utilizing the excellent property of implicit neural representation for continuous mapping. Experiments conducted on both synthetic and in-vivo data demonstrate that our proposed method surpasses other CEST denoising methods regarding both qualitative and quantitative performance.
Computation-Efficient Semi-Supervised Learning for ECG-based Cardiovascular Diseases Detection
Jun 20, 2024Abstract:Label scarcity problem is the main challenge that hinders the wide application of deep learning systems in automatic cardiovascular diseases (CVDs) detection using electrocardiography (ECG). Tuning pre-trained models alleviates this problem by transferring knowledge learned from large datasets to downstream small datasets. However, bottlenecks in computational efficiency and CVDs detection performance limit its clinical applications. It is difficult to improve the detection performance without significantly sacrificing model computational efficiency. Here, we propose a computation-efficient semi-supervised learning paradigm (FastECG) for robust and computation-efficient CVDs detection using ECG. It enables a robust adaptation of pre-trained models on downstream datasets with limited supervision and high computational efficiency. First, a random-deactivation technique is developed to achieve robust and fast low-rank adaptation of pre-trained weights. Subsequently, we propose a one-shot rank allocation module to determine the optimal ranks for the update matrices of the pre-trained weights. Finally, a lightweight semi-supervised learning pipeline is introduced to enhance model performance by leveraging labeled and unlabeled data with high computational efficiency. Extensive experiments on four downstream ECG datasets demonstrate that FastECG not only outperforms the state-of-the-art methods in multi-label CVDs detection but also consumes fewer GPU footprints, training time, and parameter storage space. As such, this paradigm provides an effective solution for achieving high computational efficiency and robust detection performance in the clinical applications of pre-trained models under limited supervision.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge