Kajsa Møllersen
Department of Community Medicine, UiT The Arctic University of Norway, Tromsø, Norway
Automating tumor-infiltrating lymphocyte assessment in breast cancer histopathology images using QuPath: a transparent and accessible machine learning pipeline
Apr 23, 2025Abstract:In this study, we built an end-to-end tumor-infiltrating lymphocytes (TILs) assessment pipeline within QuPath, demonstrating the potential of easily accessible tools to perform complex tasks in a fully automatic fashion. First, we trained a pixel classifier to segment tumor, tumor-associated stroma, and other tissue compartments in breast cancer H&E-stained whole-slide images (WSI) to isolate tumor-associated stroma for subsequent analysis. Next, we applied a pre-trained StarDist deep learning model in QuPath for cell detection and used the extracted cell features to train a binary classifier distinguishing TILs from other cells. To evaluate our TILs assessment pipeline, we calculated the TIL density in each WSI and categorized them as low, medium, or high TIL levels. Our pipeline was evaluated against pathologist-assigned TIL scores, achieving a Cohen's kappa of 0.71 on the external test set, corroborating previous research findings. These results confirm that existing software can offer a practical solution for the assessment of TILs in H&E-stained WSIs of breast cancer.
Open-source framework for detecting bias and overfitting for large pathology images
Mar 03, 2025Abstract:Even foundational models that are trained on datasets with billions of data samples may develop shortcuts that lead to overfitting and bias. Shortcuts are non-relevant patterns in data, such as the background color or color intensity. So, to ensure the robustness of deep learning applications, there is a need for methods to detect and remove such shortcuts. Today's model debugging methods are time consuming since they often require customization to fit for a given model architecture in a specific domain. We propose a generalized, model-agnostic framework to debug deep learning models. We focus on the domain of histopathology, which has very large images that require large models - and therefore large computation resources. It can be run on a workstation with a commodity GPU. We demonstrate that our framework can replicate non-image shortcuts that have been found in previous work for self-supervised learning models, and we also identify possible shortcuts in a foundation model. Our easy to use tests contribute to the development of more reliable, accurate, and generalizable models for WSI analysis. Our framework is available as an open-source tool available on github.
A Lightweight and Extensible Cell Segmentation and Classification Model for Whole Slide Images
Feb 26, 2025
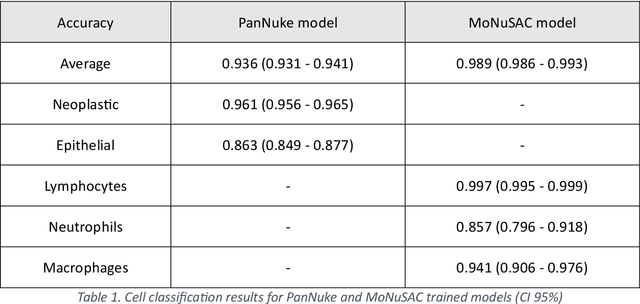

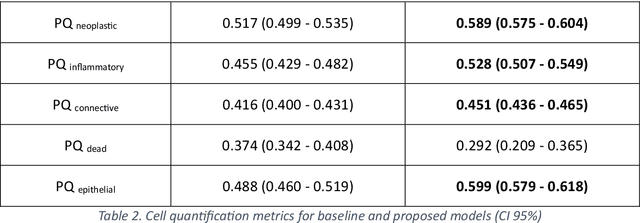
Abstract:Developing clinically useful cell-level analysis tools in digital pathology remains challenging due to limitations in dataset granularity, inconsistent annotations, high computational demands, and difficulties integrating new technologies into workflows. To address these issues, we propose a solution that enhances data quality, model performance, and usability by creating a lightweight, extensible cell segmentation and classification model. First, we update data labels through cross-relabeling to refine annotations of PanNuke and MoNuSAC, producing a unified dataset with seven distinct cell types. Second, we leverage the H-Optimus foundation model as a fixed encoder to improve feature representation for simultaneous segmentation and classification tasks. Third, to address foundation models' computational demands, we distill knowledge to reduce model size and complexity while maintaining comparable performance. Finally, we integrate the distilled model into QuPath, a widely used open-source digital pathology platform. Results demonstrate improved segmentation and classification performance using the H-Optimus-based model compared to a CNN-based model. Specifically, average $R^2$ improved from 0.575 to 0.871, and average $PQ$ score improved from 0.450 to 0.492, indicating better alignment with actual cell counts and enhanced segmentation quality. The distilled model maintains comparable performance while reducing parameter count by a factor of 48. By reducing computational complexity and integrating into workflows, this approach may significantly impact diagnostics, reduce pathologist workload, and improve outcomes. Although the method shows promise, extensive validation is necessary prior to clinical deployment.
Fast TILs estimation in lung cancer WSIs based on semi-stochastic patch sampling
May 05, 2024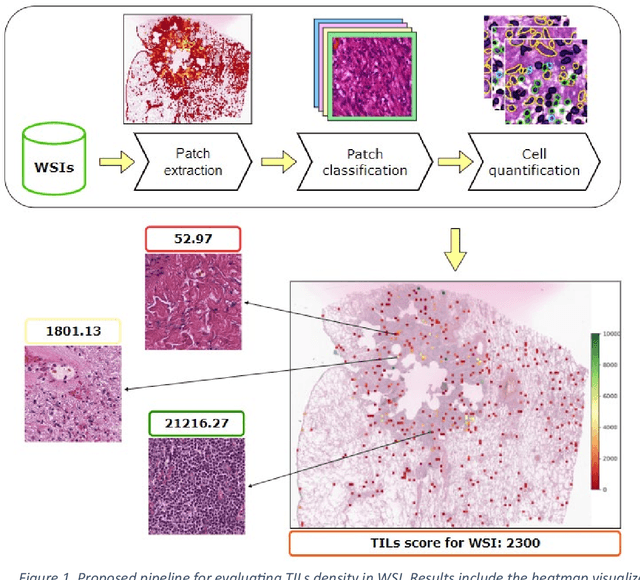
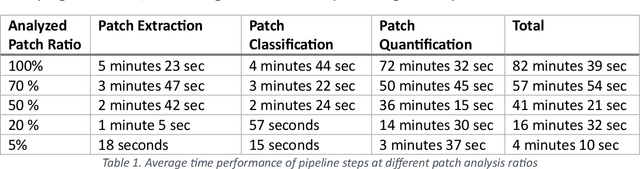
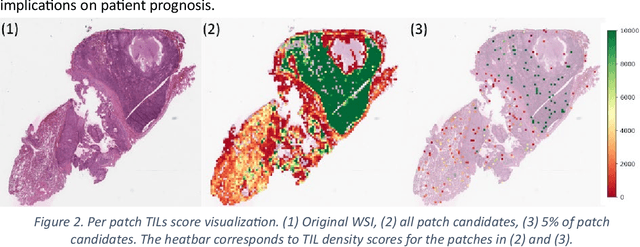
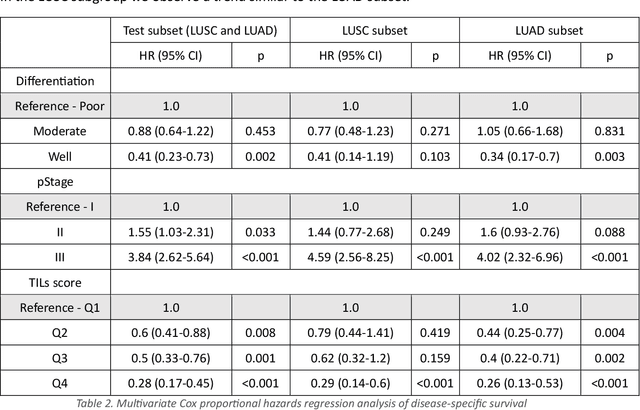
Abstract:Addressing the critical need for accurate prognostic biomarkers in cancer treatment, quantifying tumor-infiltrating lymphocytes (TILs) in non-small cell lung cancer (NSCLC) presents considerable challenges. Manual TIL quantification in whole slide images (WSIs) is laborious and subject to variability, potentially undermining patient outcomes. Our study introduces an automated pipeline that utilizes semi-stochastic patch sampling, patch classification to retain prognostically relevant patches, and cell quantification using the HoVer-Net model to streamline the TIL evaluation process. This pipeline efficiently excludes approximately 70% of areas not relevant for prognosis and requires only 5% of the remaining patches to maintain prognostic accuracy (c-index 0.65 +- 0.01). The computational efficiency achieved does not sacrifice prognostic accuracy, as demonstrated by the TILs score's strong correlation with patient survival, which surpasses traditional CD8 IHC scoring methods. While the pipeline demonstrates potential for enhancing NSCLC prognostication and personalization of treatment, comprehensive clinical validation is still required. Future research should focus on verifying its broader clinical utility and investigating additional biomarkers to improve NSCLC prognosis.
Publicly available datasets of breast histopathology H&E whole-slide images: A systematic review
Jun 02, 2023Abstract:Advancements in digital pathology and computing resources have made a significant impact in the field of computational pathology for breast cancer diagnosis and treatment. However, access to high-quality labeled histopathological images of breast cancer is a big challenge that limits the development of accurate and robust deep learning models. In this systematic review, we identified the publicly available datasets of breast H&E stained whole-slide images (WSI) that can be used to develop deep learning algorithms. We systematically searched nine scientific literature databases and nine research data repositories. We found twelve publicly available datasets, containing 5153 H&E WSIs of breast cancer. Moreover, we reported image metadata and characteristics for each dataset to assist researchers in selecting proper datasets for specific tasks in breast cancer computational pathology. In addition, we compiled a list of patch and private datasets that were used in the included articles as a supplementary resource for researchers. Notably, 22% of the included articles utilized multiple datasets, and only 12% of the articles used an external validation set, suggesting that the performance of other developed models may be susceptible to overestimation. The TCGA-BRCA was used in 47.4% of the selected studies. This dataset has a considerable selection bias that can impact the robustness and generalizability of the trained algorithms. There is also a lack of consistent metadata reporting of breast WSI datasets that can be an issue in developing accurate deep learning models, indicating the necessity of establishing explicit guidelines for documenting breast WSI dataset characteristics and metadata.
What is the state of the art? Accounting for multiplicity in machine learning benchmark performance
Mar 10, 2023Abstract:Machine learning methods are commonly evaluated and compared by their performance on data sets from public repositories. This allows for multiple methods, oftentimes several thousands, to be evaluated under identical conditions and across time. The highest ranked performance on a problem is referred to as state-of-the-art (SOTA) performance, and is used, among other things, as a reference point for publication of new methods. Using the highest-ranked performance as an estimate for SOTA is a biased estimator, giving overly optimistic results. The mechanisms at play are those of multiplicity, a topic that is well-studied in the context of multiple comparisons and multiple testing, but has, as far as the authors are aware of, been nearly absent from the discussion regarding SOTA estimates. The optimistic state-of-the-art estimate is used as a standard for evaluating new methods, and methods with substantial inferior results are easily overlooked. In this article, we provide a probability distribution for the case of multiple classifiers so that known analyses methods can be engaged and a better SOTA estimate can be provided. We demonstrate the impact of multiplicity through a simulated example with independent classifiers. We show how classifier dependency impacts the variance, but also that the impact is limited when the accuracy is high. Finally, we discuss a real-world example; a Kaggle competition from 2020.
A Pragmatic Machine Learning Approach to Quantify Tumor Infiltrating Lymphocytes in Whole Slide Images
Feb 14, 2022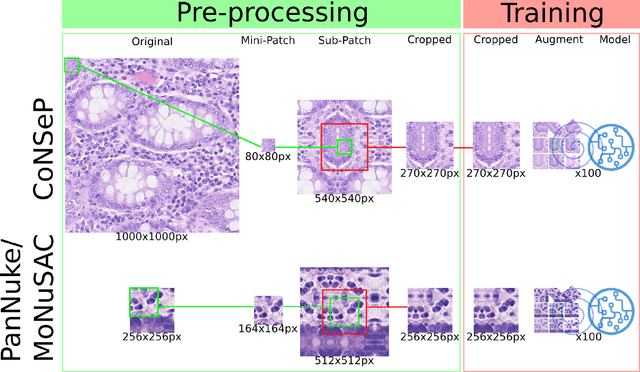
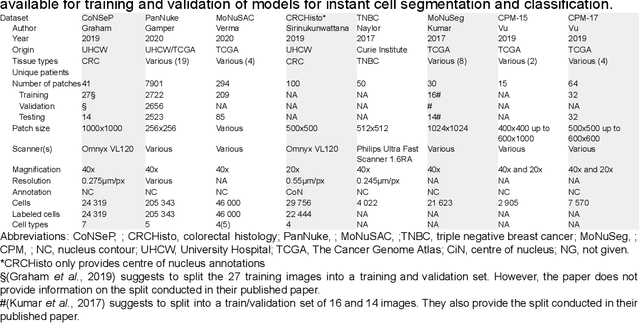
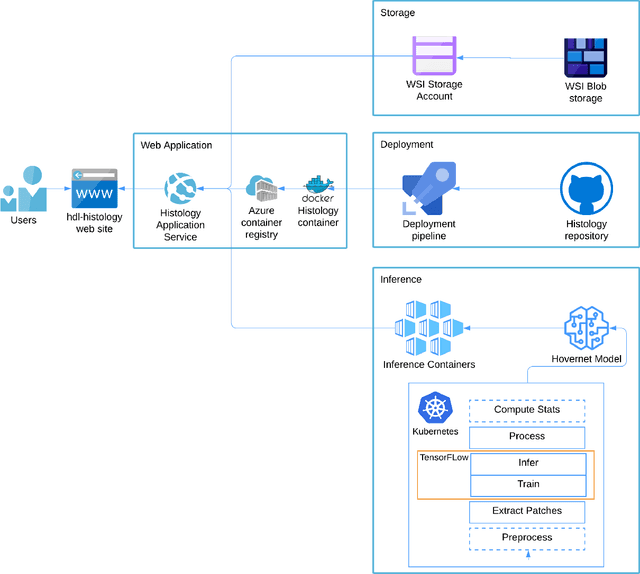
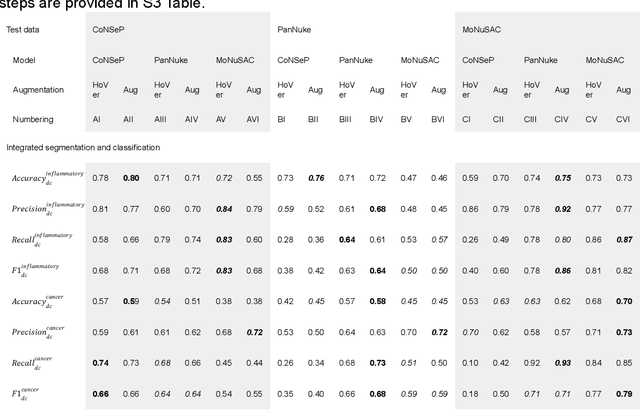
Abstract:Increased levels of tumor infiltrating lymphocytes (TILs) in cancer tissue indicate favourable outcomes in many types of cancer. Manual quantification of immune cells is inaccurate and time consuming for pathologists. Our aim is to leverage a computational solution to automatically quantify TILs in whole slide images (WSIs) of standard diagnostic haematoxylin and eosin stained sections (H&E slides) from lung cancer patients. Our approach is to transfer an open source machine learning method for segmentation and classification of nuclei in H&E slides trained on public data to TIL quantification without manual labeling of our data. Our results show that additional augmentation improves model transferability when training on few samples/limited tissue types. Models trained with sufficient samples/tissue types do not benefit from our additional augmentation policy. Further, the resulting TIL quantification correlates to patient prognosis and compares favorably to the current state-of-the-art method for immune cell detection in non-small lung cancer (current standard CD8 cells in DAB stained TMAs HR 0.34 95% CI 0.17-0.68 vs TILs in HE WSIs: HoVer-Net PanNuke Aug Model HR 0.30 95% CI 0.15-0.60, HoVer-Net MoNuSAC Aug model HR 0.27 95% CI 0.14-0.53). Moreover, we implemented a cloud based system to train, deploy and visually inspect machine learning based annotation for H&E slides. Our pragmatic approach bridges the gap between machine learning research, translational clinical research and clinical implementation. However, validation in prospective studies is needed to assert that the method works in a clinical setting.
Instance Segmentation of Microscopic Foraminifera
May 15, 2021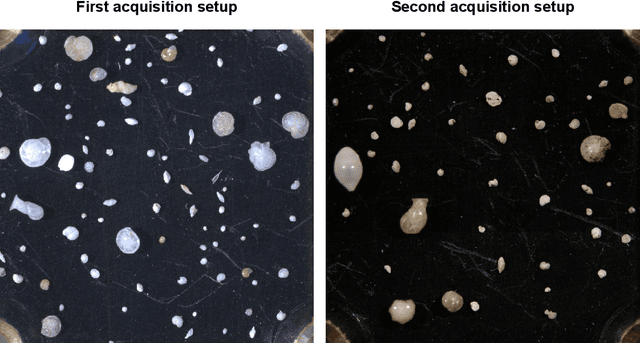
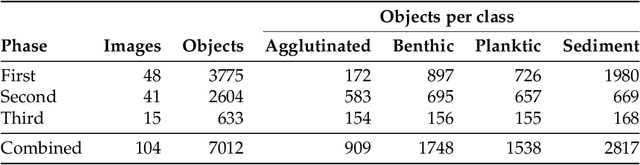
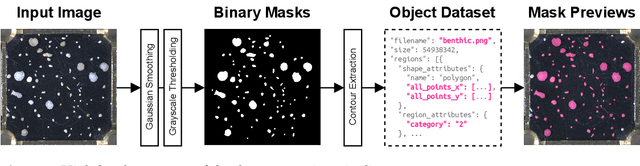
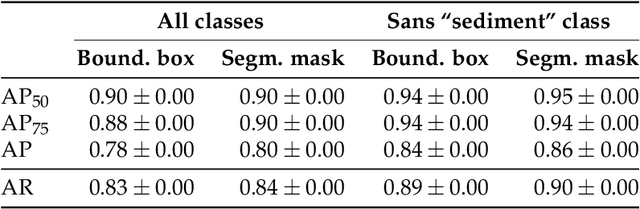
Abstract:Foraminifera are single-celled marine organisms that construct shells that remain as fossils in the marine sediments. Classifying and counting these fossils are important in e.g. paleo-oceanographic and -climatological research. However, the identification and counting process has been performed manually since the 1800s and is laborious and time-consuming. In this work, we present a deep learning-based instance segmentation model for classifying, detecting, and segmenting microscopic foraminifera. Our model is based on the Mask R-CNN architecture, using model weight parameters that have learned on the COCO detection dataset. We use a fine-tuning approach to adapt the parameters on a novel object detection dataset of more than 7000 microscopic foraminifera and sediment grains. The model achieves a (COCO-style) average precision of $0.78 \pm 0.00$ on the classification and detection task, and $0.80 \pm 0.00$ on the segmentation task. When the model is evaluated without challenging sediment grain images, the average precision for both tasks increases to $0.84 \pm 0.00$ and $0.86 \pm 0.00$, respectively. Prediction results are analyzed both quantitatively and qualitatively and discussed. Based on our findings we propose several directions for future work, and conclude that our proposed model is an important step towards automating the identification and counting of microscopic foraminifera.
A bag-to-class divergence approach to multiple-instance learning
Oct 12, 2018
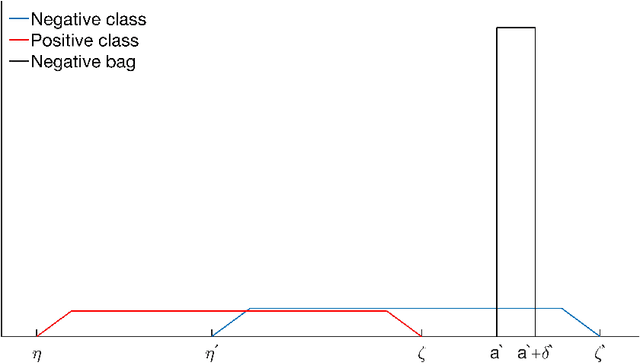
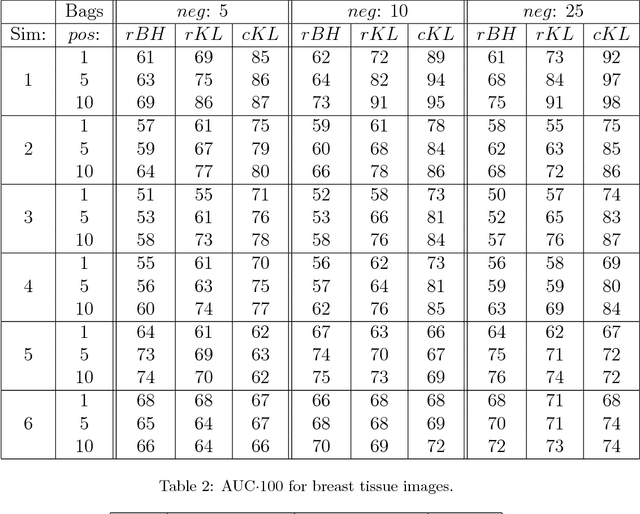
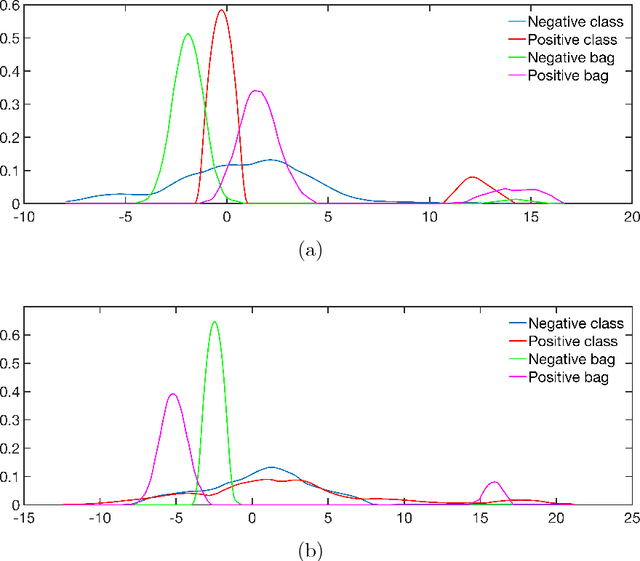
Abstract:In multi-instance (MI) learning, each object (bag) consists of multiple feature vectors (instances), and is most commonly regarded as a set of points in a multidimensional space. A different viewpoint is that the instances are realisations of random vectors with corresponding probability distribution, and that a bag is the distribution, not the realisations. In MI classification, each bag in the training set has a class label, but the instances are unlabelled. By introducing the probability distribution space to bag-level classification problems, dissimilarities between probability distributions (divergences) can be applied. The bag-to-bag Kullback-Leibler information is asymptotically the best classifier, but the typical sparseness of MI training sets is an obstacle. We introduce bag-to-class divergence to MI learning, emphasising the hierarchical nature of the random vectors that makes bags from the same class different. We propose two properties for bag-to-class divergences, and an additional property for sparse training sets.
Replication study: Development and validation of deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs
Aug 30, 2018
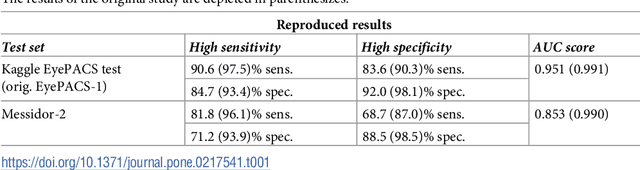
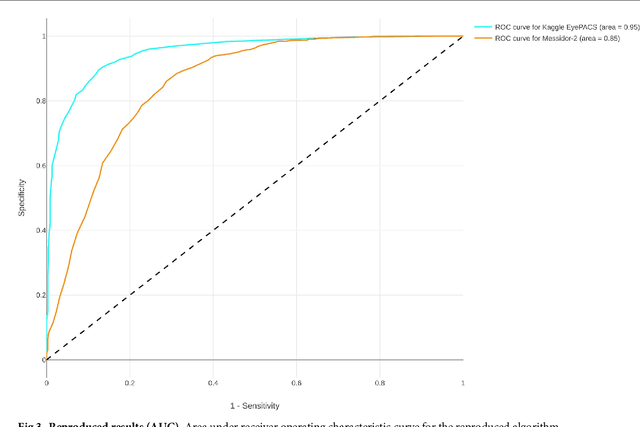
Abstract:Replication studies are essential for validation of new methods, and are crucial to maintain the high standards of scientific publications, and to use the results in practice. We have attempted to replicate the main method in 'Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs' published in JAMA 2016; 316(22). We re-implemented the method since the source code is not available, and we used publicly available data sets. The original study used non-public fundus images from EyePACS and three hospitals in India for training. We used a different EyePACS data set from Kaggle. The original study used the benchmark data set Messidor-2 to evaluate the algorithm's performance. We used the same data set. In the original study, ophthalmologists re-graded all images for diabetic retinopathy, macular edema, and image gradability. There was one diabetic retinopathy grade per image for our data sets, and we assessed image gradability ourselves. Hyper-parameter settings were not described in the original study. But some of these were later published. We were not able to replicate the original study. Our algorithm's area under the receiver operating curve (AUC) of 0.94 on the Kaggle EyePACS test set and 0.80 on Messidor-2 did not come close to the reported AUC of 0.99 in the original study. This may be caused by the use of a single grade per image, different data, or different not described hyper-parameter settings. This study shows the challenges of replicating deep learning, and the need for more replication studies to validate deep learning methods, especially for medical image analysis. Our source code and instructions are available at: https://github.com/mikevoets/jama16-retina-replication
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge