Junhua Zheng
Artificial intelligence for diagnosing and predicting survival of patients with renal cell carcinoma: Retrospective multi-center study
Jan 12, 2023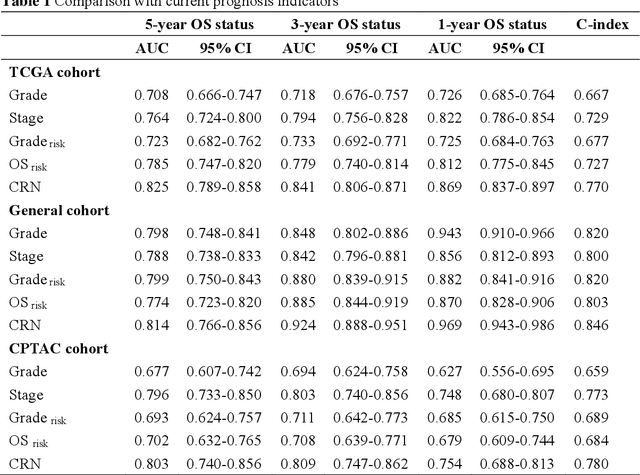
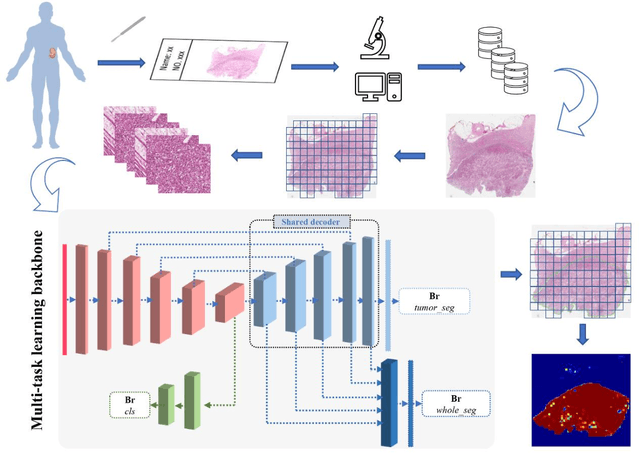
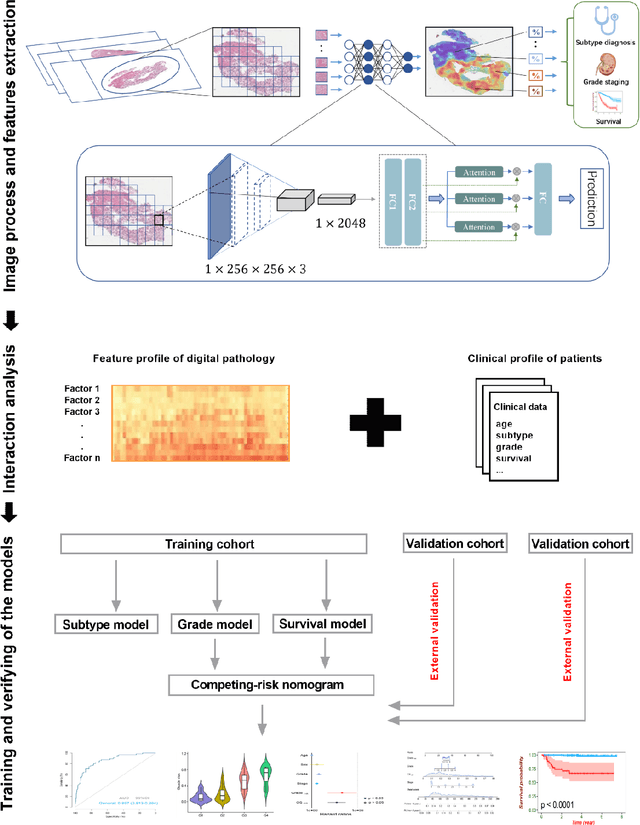
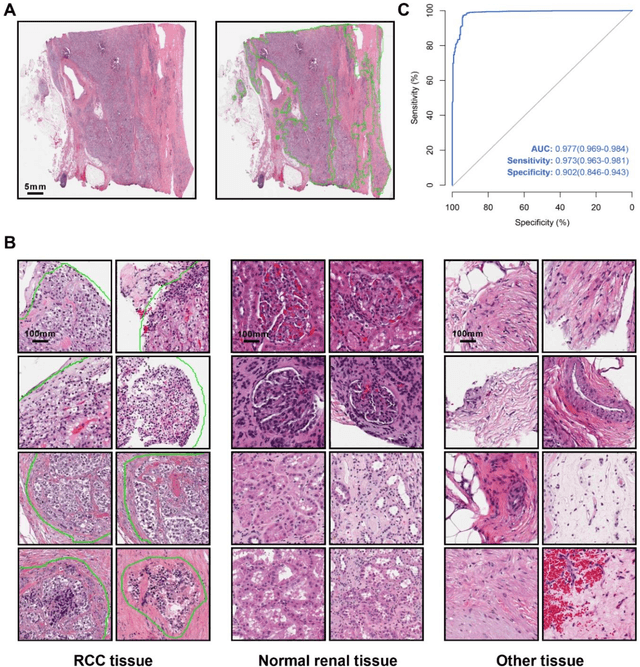
Abstract:Background: Clear cell renal cell carcinoma (ccRCC) is the most common renal-related tumor with high heterogeneity. There is still an urgent need for novel diagnostic and prognostic biomarkers for ccRCC. Methods: We proposed a weakly-supervised deep learning strategy using conventional histology of 1752 whole slide images from multiple centers. Our study was demonstrated through internal cross-validation and external validations for the deep learning-based models. Results: Automatic diagnosis for ccRCC through intelligent subtyping of renal cell carcinoma was proved in this study. Our graderisk achieved aera the curve (AUC) of 0.840 (95% confidence interval: 0.805-0.871) in the TCGA cohort, 0.840 (0.805-0.871) in the General cohort, and 0.840 (0.805-0.871) in the CPTAC cohort for the recognition of high-grade tumor. The OSrisk for the prediction of 5-year survival status achieved AUC of 0.784 (0.746-0.819) in the TCGA cohort, which was further verified in the independent General cohort and the CPTAC cohort, with AUC of 0.774 (0.723-0.820) and 0.702 (0.632-0.765), respectively. Cox regression analysis indicated that graderisk, OSrisk, tumor grade, and tumor stage were found to be independent prognostic factors, which were further incorporated into the competing-risk nomogram (CRN). Kaplan-Meier survival analyses further illustrated that our CRN could significantly distinguish patients with high survival risk, with hazard ratio of 5.664 (3.893-8.239, p < 0.0001) in the TCGA cohort, 35.740 (5.889-216.900, p < 0.0001) in the General cohort and 6.107 (1.815 to 20.540, p < 0.0001) in the CPTAC cohort. Comparison analyses conformed that our CRN outperformed current prognosis indicators in the prediction of survival status, with higher concordance index for clinical prognosis.
Pan-cancer computational histopathology reveals tumor mutational burden status through weakly-supervised deep learning
Apr 07, 2022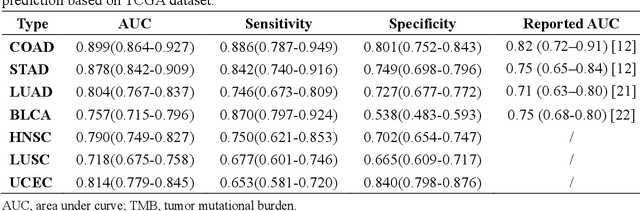
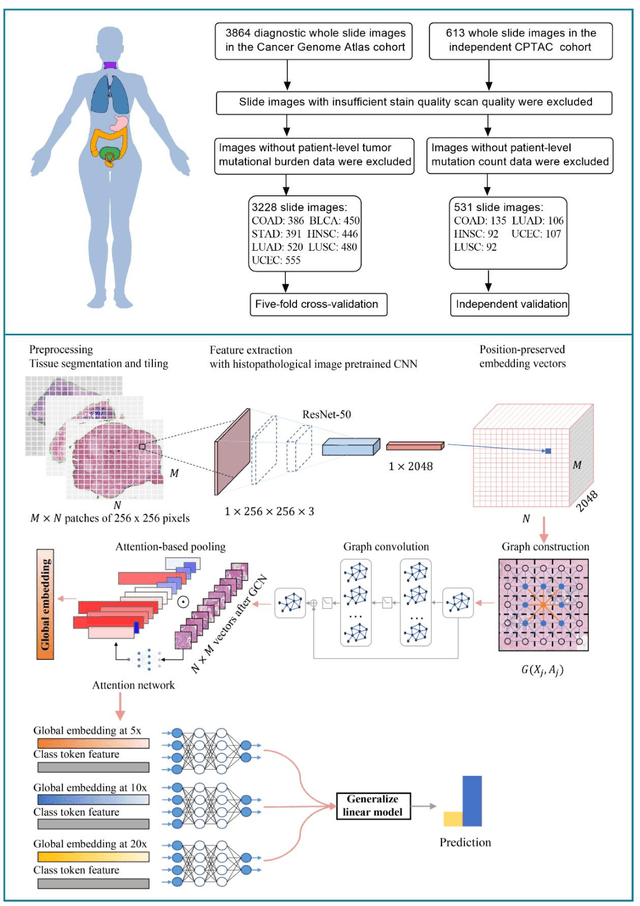
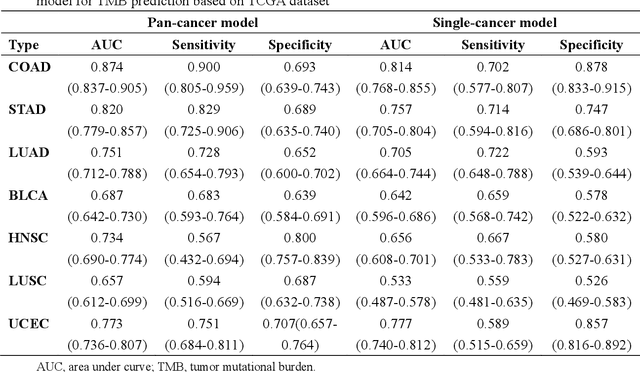
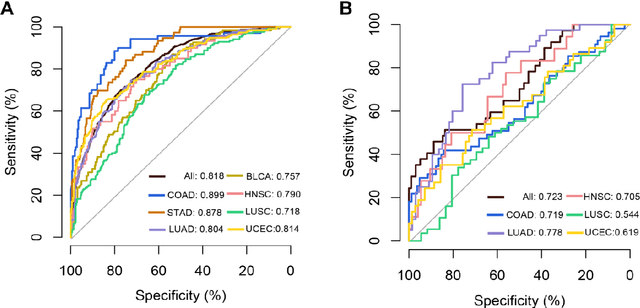
Abstract:Tumor mutational burden (TMB) is a potential genomic biomarker that can help identify patients who will benefit from immunotherapy across a variety of cancers. We included whole slide images (WSIs) of 3228 diagnostic slides from the Cancer Genome Atlas and 531 WSIs from the Clinical Proteomic Tumor Analysis Consortium for the development and verification of a pan-cancer TMB prediction model (PC-TMB). We proposed a multiscale weakly-supervised deep learning framework for predicting TMB of seven types of tumors based only on routinely used hematoxylin-eosin (H&E)-stained WSIs. PC-TMB achieved a mean area under curve (AUC) of 0.818 (0.804-0.831) in the cross-validation cohort, which was superior to the best single-scale model. In comparison with the state-of-the-art TMB prediction model from previous publications, our multiscale model achieved better performance over previously reported models. In addition, the improvements of PC-TMB over the single-tumor models were also confirmed by the ablation tests on 10x magnification. The PC-TMB algorithm also exhibited good generalization on external validation cohort with AUC of 0.732 (0.683-0.761). PC-TMB possessed a comparable survival-risk stratification performance to the TMB measured by whole exome sequencing, but with low cost and being time-efficient for providing a prognostic biomarker of multiple solid tumors. Moreover, spatial heterogeneity of TMB within tumors was also identified through our PC-TMB, which might enable image-based screening for molecular biomarkers with spatial variation and potential exploring for genotype-spatial heterogeneity relationships.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge