Julia Vogt
University of Basel
scSSL-Bench: Benchmarking Self-Supervised Learning for Single-Cell Data
Jun 10, 2025Abstract:Self-supervised learning (SSL) has proven to be a powerful approach for extracting biologically meaningful representations from single-cell data. To advance our understanding of SSL methods applied to single-cell data, we present scSSL-Bench, a comprehensive benchmark that evaluates nineteen SSL methods. Our evaluation spans nine datasets and focuses on three common downstream tasks: batch correction, cell type annotation, and missing modality prediction. Furthermore, we systematically assess various data augmentation strategies. Our analysis reveals task-specific trade-offs: the specialized single-cell frameworks, scVI, CLAIRE, and the finetuned scGPT excel at uni-modal batch correction, while generic SSL methods, such as VICReg and SimCLR, demonstrate superior performance in cell typing and multi-modal data integration. Random masking emerges as the most effective augmentation technique across all tasks, surpassing domain-specific augmentations. Notably, our results indicate the need for a specialized single-cell multi-modal data integration framework. scSSL-Bench provides a standardized evaluation platform and concrete recommendations for applying SSL to single-cell analysis, advancing the convergence of deep learning and single-cell genomics.
Structured Generations: Using Hierarchical Clusters to guide Diffusion Models
Jul 08, 2024Abstract:This paper introduces Diffuse-TreeVAE, a deep generative model that integrates hierarchical clustering into the framework of Denoising Diffusion Probabilistic Models (DDPMs). The proposed approach generates new images by sampling from a root embedding of a learned latent tree VAE-based structure, it then propagates through hierarchical paths, and utilizes a second-stage DDPM to refine and generate distinct, high-quality images for each data cluster. The result is a model that not only improves image clarity but also ensures that the generated samples are representative of their respective clusters, addressing the limitations of previous VAE-based methods and advancing the state of clustering-based generative modeling.
Tree Variational Autoencoders
Jul 01, 2023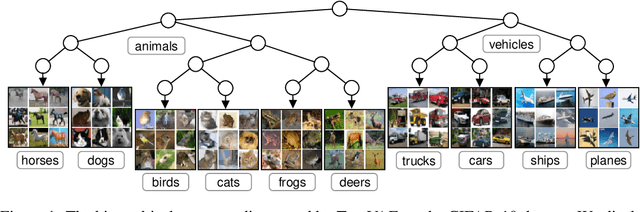
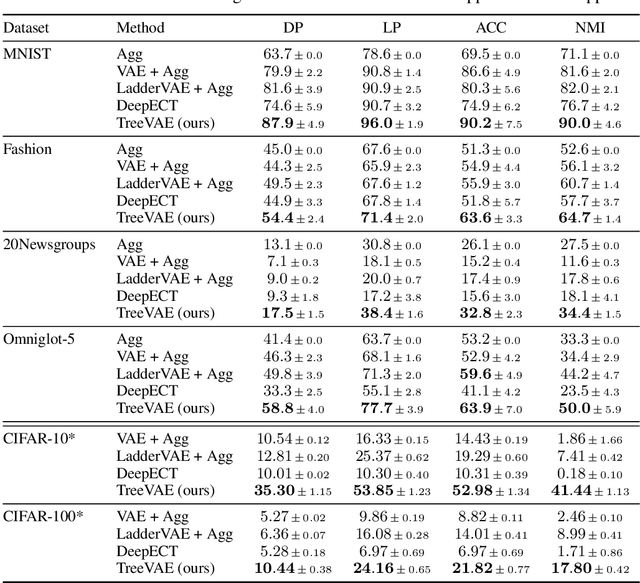
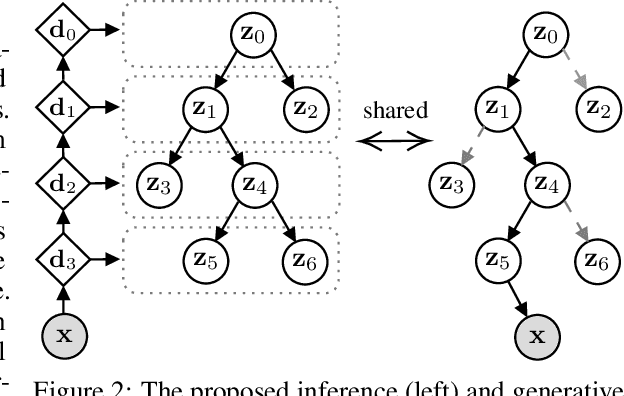
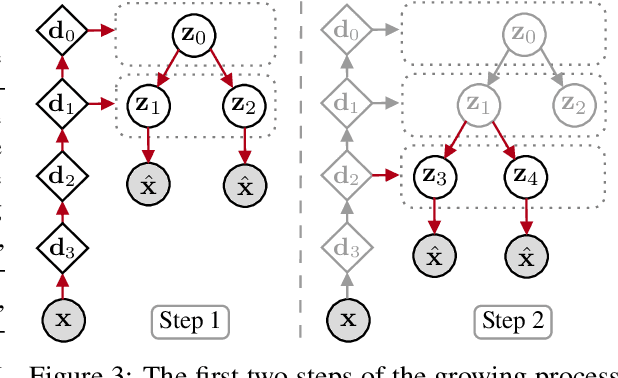
Abstract:We propose a new generative hierarchical clustering model that learns a flexible tree-based posterior distribution over latent variables. The proposed Tree Variational Autoencoder (TreeVAE) hierarchically divides samples according to their intrinsic characteristics, shedding light on hidden structure in the data. It adapts its architecture to discover the optimal tree for encoding dependencies between latent variables. The proposed tree-based generative architecture permits lightweight conditional inference and improves generative performance by utilizing specialized leaf decoders. We show that TreeVAE uncovers underlying clusters in the data and finds meaningful hierarchical relations between the different groups on a variety of datasets, including real-world imaging data. We present empirically that TreeVAE provides a more competitive log-likelihood lower bound than the sequential counterparts. Finally, due to its generative nature, TreeVAE is able to generate new samples from the discovered clusters via conditional sampling.
Interpretable Prediction of Pulmonary Hypertension in Newborns using Echocardiograms
Mar 24, 2022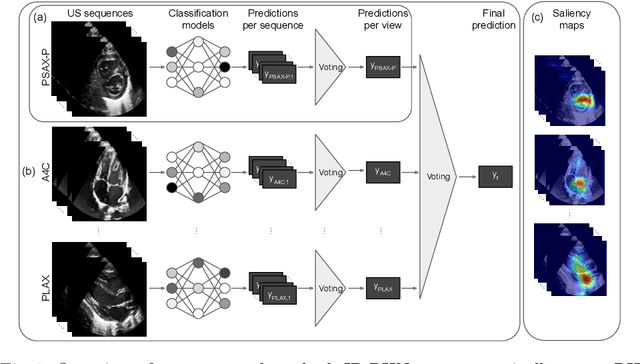
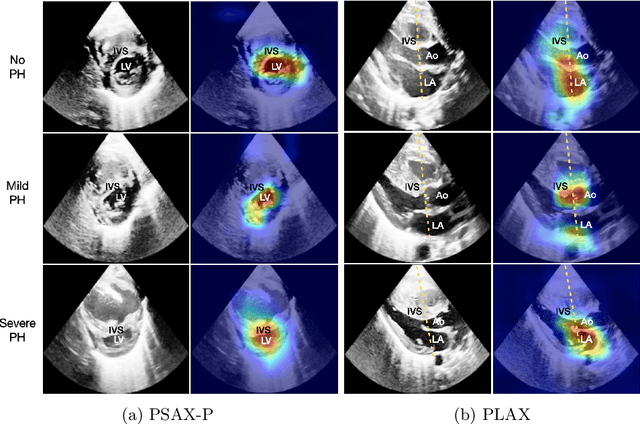
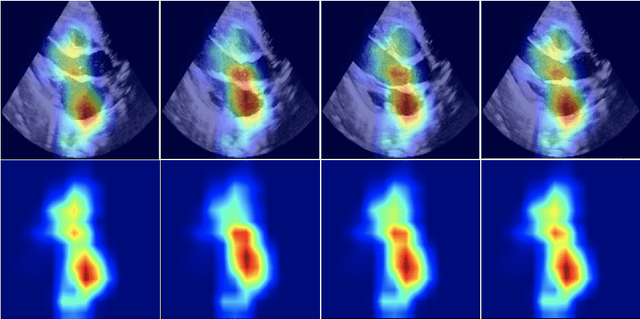
Abstract:Pulmonary hypertension (PH) in newborns and infants is a complex condition associated with several pulmonary, cardiac, and systemic diseases contributing to morbidity and mortality. Therefore, accurate and early detection of PH is crucial for successful management. Using echocardiography, the primary diagnostic tool in pediatrics, human assessment is both time-consuming and expertise-demanding, raising the need for an automated approach. In this work, we present an interpretable multi-view video-based deep learning approach to predict PH for a cohort of 194 newborns using echocardiograms. We use spatio-temporal convolutional architectures for the prediction of PH from each view, and aggregate the predictions of the different views using majority voting. To the best of our knowledge, this is the first work for an automated assessment of PH in newborns using echocardiograms. Our results show a mean F1-score of 0.84 for severity prediction and 0.92 for binary detection using 10-fold cross-validation. We complement our predictions with saliency maps and show that the learned model focuses on clinically relevant cardiac structures, motivating its usage in clinical practice.
A Complete Analysis of the l_1,p Group-Lasso
Jun 18, 2012



Abstract:The Group-Lasso is a well-known tool for joint regularization in machine learning methods. While the l_{1,2} and the l_{1,\infty} version have been studied in detail and efficient algorithms exist, there are still open questions regarding other l_{1,p} variants. We characterize conditions for solutions of the l_{1,p} Group-Lasso for all p-norms with 1 <= p <= \infty, and we present a unified active set algorithm. For all p-norms, a highly efficient projected gradient algorithm is presented. This new algorithm enables us to compare the prediction performance of many variants of the Group-Lasso in a multi-task learning setting, where the aim is to solve many learning problems in parallel which are coupled via the Group-Lasso constraint. We conduct large-scale experiments on synthetic data and on two real-world data sets. In accordance with theoretical characterizations of the different norms we observe that the weak-coupling norms with p between 1.5 and 2 consistently outperform the strong-coupling norms with p >> 2.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge