Joshua Durso-Finley
Probabilistic Temporal Prediction of Continuous Disease Trajectories and Treatment Effects Using Neural SDEs
Jun 18, 2024



Abstract:Personalized medicine based on medical images, including predicting future individualized clinical disease progression and treatment response, would have an enormous impact on healthcare and drug development, particularly for diseases (e.g. multiple sclerosis (MS)) with long term, complex, heterogeneous evolutions and no cure. In this work, we present the first stochastic causal temporal framework to model the continuous temporal evolution of disease progression via Neural Stochastic Differential Equations (NSDE). The proposed causal inference model takes as input the patient's high dimensional images (MRI) and tabular data, and predicts both factual and counterfactual progression trajectories on different treatments in latent space. The NSDE permits the estimation of high-confidence personalized trajectories and treatment effects. Extensive experiments were performed on a large, multi-centre, proprietary dataset of patient 3D MRI and clinical data acquired during several randomized clinical trials for MS treatments. Our results present the first successful uncertainty-based causal Deep Learning (DL) model to: (a) accurately predict future patient MS disability evolution (e.g. EDSS) and treatment effects leveraging baseline MRI, and (b) permit the discovery of subgroups of patients for which the model has high confidence in their response to treatment even in clinical trials which did not reach their clinical endpoints.
Improving Image-Based Precision Medicine with Uncertainty-Aware Causal Models
May 05, 2023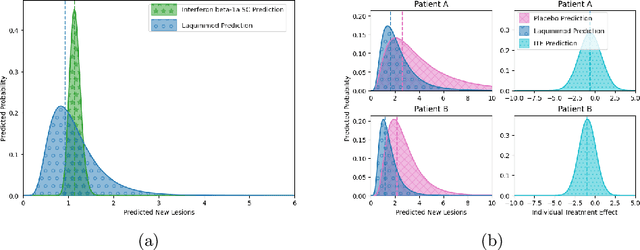
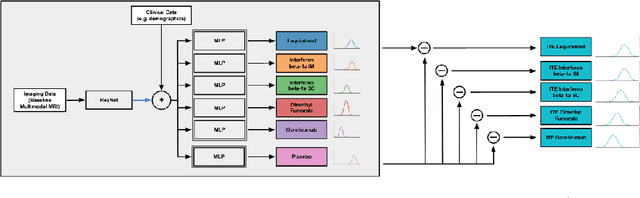
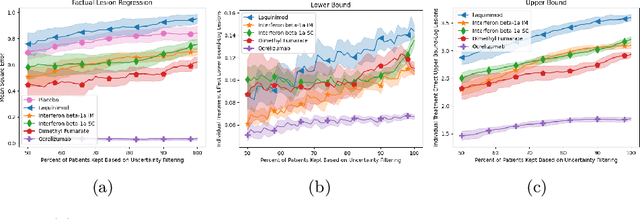
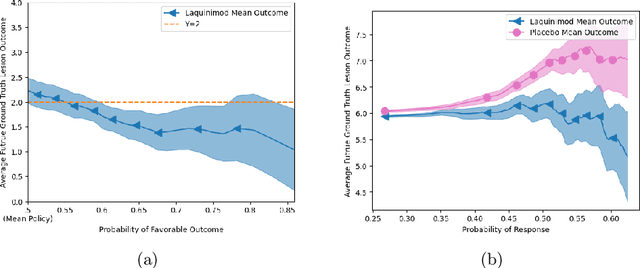
Abstract:Image-based precision medicine aims to personalize treatment decisions based on an individual's unique imaging features so as to improve their clinical outcome. Machine learning frameworks that integrate uncertainty estimation as part of their treatment recommendations would be safer and more reliable. However, little work has been done in adapting uncertainty estimation techniques and validation metrics for precision medicine. In this paper, we use Bayesian deep learning for estimating the posterior distribution over factual and counterfactual outcomes on several treatments. This allows for estimating the uncertainty for each treatment option and for the individual treatment effects (ITE) between any two treatments. We train and evaluate this model to predict future new and enlarging T2 lesion counts on a large, multi-center dataset of MR brain images of patients with multiple sclerosis, exposed to several treatments during randomized controlled trials. We evaluate the correlation of the uncertainty estimate with the factual error, and, given the lack of ground truth counterfactual outcomes, demonstrate how uncertainty for the ITE prediction relates to bounds on the ITE error. Lastly, we demonstrate how knowledge of uncertainty could modify clinical decision-making to improve individual patient and clinical trial outcomes.
Personalized Prediction of Future Lesion Activity and Treatment Effect in Multiple Sclerosis from Baseline MRI
Apr 01, 2022



Abstract:Precision medicine for chronic diseases such as multiple sclerosis (MS) involves choosing a treatment which best balances efficacy and side effects/preferences for individual patients. Making this choice as early as possible is important, as delays in finding an effective therapy can lead to irreversible disability accrual. To this end, we present the first deep neural network model for individualized treatment decisions from baseline magnetic resonance imaging (MRI) (with clinical information if available) for MS patients. Our model (a) predicts future new and enlarging T2 weighted (NE-T2) lesion counts on follow-up MRI on multiple treatments and (b) estimates the conditional average treatment effect (CATE), as defined by the predicted future suppression of NE-T2 lesions, between different treatment options relative to placebo. Our model is validated on a proprietary federated dataset of 1817 multi-sequence MRIs acquired from MS patients during four multi-centre randomized clinical trials. Our framework achieves high average precision in the binarized regression of future NE-T2 lesions on five different treatments, identifies heterogeneous treatment effects, and provides a personalized treatment recommendation that accounts for treatment-associated risk (e.g. side effects, patient preference, administration difficulties).
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge