Joaquim Salvi
Benchmarking the CoW with the TopCoW Challenge: Topology-Aware Anatomical Segmentation of the Circle of Willis for CTA and MRA
Dec 29, 2023



Abstract:The Circle of Willis (CoW) is an important network of arteries connecting major circulations of the brain. Its vascular architecture is believed to affect the risk, severity, and clinical outcome of serious neuro-vascular diseases. However, characterizing the highly variable CoW anatomy is still a manual and time-consuming expert task. The CoW is usually imaged by two angiographic imaging modalities, magnetic resonance angiography (MRA) and computed tomography angiography (CTA), but there exist limited public datasets with annotations on CoW anatomy, especially for CTA. Therefore we organized the TopCoW Challenge in 2023 with the release of an annotated CoW dataset and invited submissions worldwide for the CoW segmentation task, which attracted over 140 registered participants from four continents. TopCoW dataset was the first public dataset with voxel-level annotations for CoW's 13 vessel components, made possible by virtual-reality (VR) technology. It was also the first dataset with paired MRA and CTA from the same patients. TopCoW challenge aimed to tackle the CoW characterization problem as a multiclass anatomical segmentation task with an emphasis on topological metrics. The top performing teams managed to segment many CoW components to Dice scores around 90%, but with lower scores for communicating arteries and rare variants. There were also topological mistakes for predictions with high Dice scores. Additional topological analysis revealed further areas for improvement in detecting certain CoW components and matching CoW variant's topology accurately. TopCoW represented a first attempt at benchmarking the CoW anatomical segmentation task for MRA and CTA, both morphologically and topologically.
Multiple Sclerosis Lesion Synthesis in MRI using an encoder-decoder U-NET
Jan 17, 2019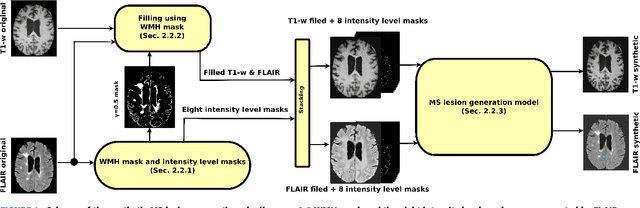
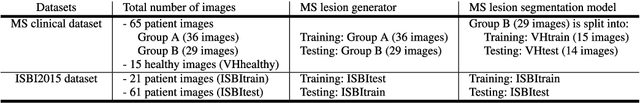

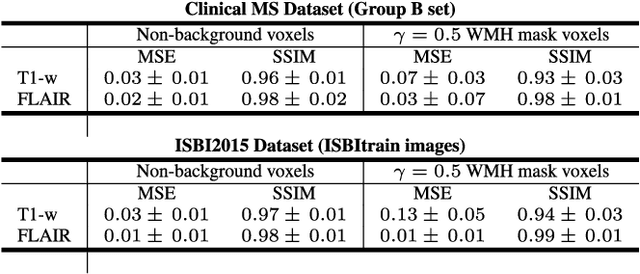
Abstract:In this paper, we propose generating synthetic multiple sclerosis (MS) lesions on MRI images with the final aim to improve the performance of supervised machine learning algorithms, therefore avoiding the problem of the lack of available ground truth. We propose a two-input two-output fully convolutional neural network model for MS lesion synthesis in MRI images. The lesion information is encoded as discrete binary intensity level masks passed to the model and stacked with the input images. The model is trained end-to-end without the need for manually annotating the lesions in the training set. We then perform the generation of synthetic lesions on healthy images via registration of patient images, which are subsequently used for data augmentation to increase the performance for supervised MS lesion detection algorithms. Our pipeline is evaluated on MS patient data from an in-house clinical dataset and the public ISBI2015 challenge dataset. The evaluation is based on measuring the similarities between the real and the synthetic images as well as in terms of lesion detection performance by segmenting both the original and synthetic images individually using a state-of-the-art segmentation framework. We also demonstrate the usage of synthetic MS lesions generated on healthy images as data augmentation. We analyze a scenario of limited training data (one-image training) to demonstrate the effect of the data augmentation on both datasets. Our results significantly show the effectiveness of the usage of synthetic MS lesion images. For the ISBI2015 challenge, our one-image model trained using only a single image plus the synthetic data augmentation strategy showed a performance similar to that of other CNN methods that were fully trained using the entire training set, yielding a comparable human expert rater performance
One-shot domain adaptation in multiple sclerosis lesion segmentation using convolutional neural networks
May 31, 2018
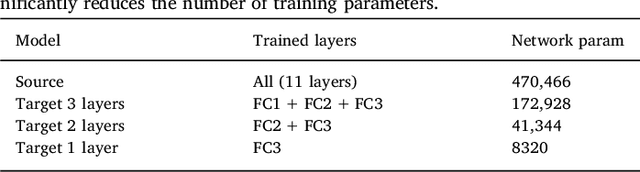
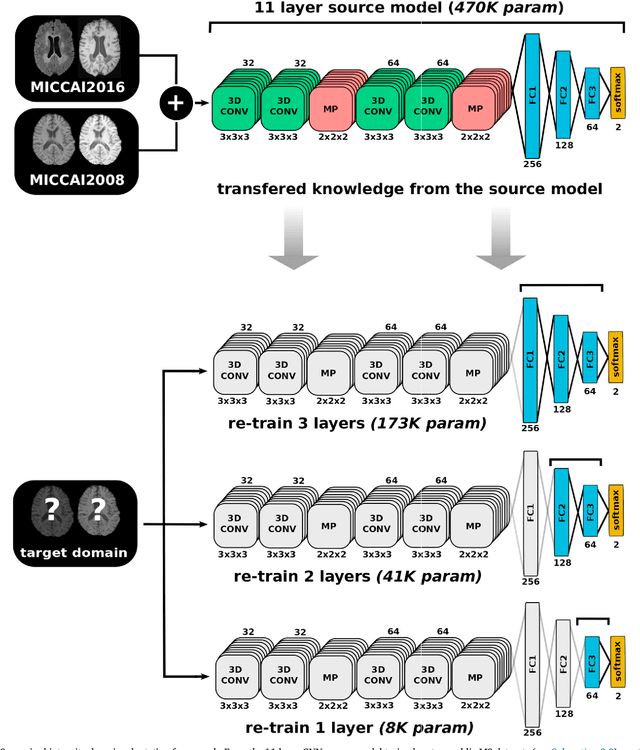
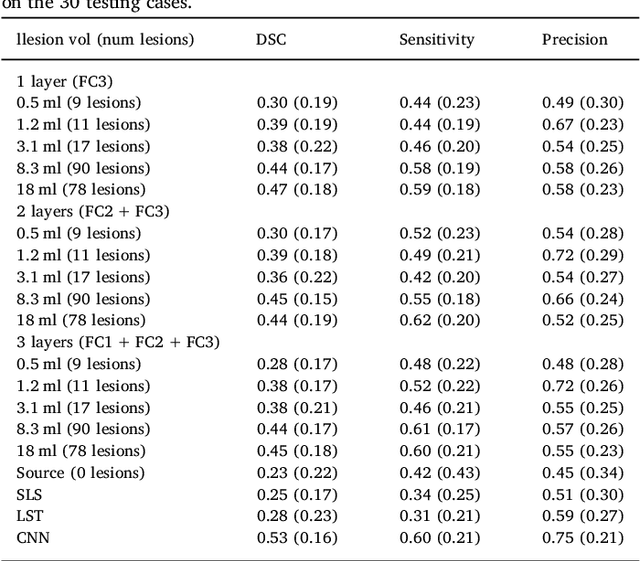
Abstract:In recent years, several convolutional neural network (CNN) methods have been proposed for the automated white matter lesion segmentation of multiple sclerosis (MS) patient images, due to their superior performance compared with those of other state-of-the-art methods. However, the accuracies of CNN methods tend to decrease significantly when evaluated on different image domains compared with those used for training, which demonstrates the lack of adaptability of CNNs to unseen imaging data. In this study, we analyzed the effect of intensity domain adaptation on our recently proposed CNN-based MS lesion segmentation method. Given a source model trained on two public MS datasets, we investigated the transferability of the CNN model when applied to other MRI scanners and protocols, evaluating the minimum number of annotated images needed from the new domain and the minimum number of layers needed to re-train to obtain comparable accuracy. Our analysis comprised MS patient data from both a clinical center and the public ISBI2015 challenge database, which permitted us to compare the domain adaptation capability of our model to that of other state-of-the-art methods. For the ISBI2015 challenge, our one-shot domain adaptation model trained using only a single image showed a performance similar to that of other CNN methods that were fully trained using the entire available training set, yielding a comparable human expert rater performance. We believe that our experiments will encourage the MS community to incorporate its use in different clinical settings with reduced amounts of annotated data. This approach could be meaningful not only in terms of the accuracy in delineating MS lesions but also in the related reductions in time and economic costs derived from manual lesion labeling.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge