Jingru Fu
Synthesizing Individualized Aging Brains in Health and Disease with Generative Models and Parallel Transport
Feb 28, 2025



Abstract:Simulating prospective magnetic resonance imaging (MRI) scans from a given individual brain image is challenging, as it requires accounting for canonical changes in aging and/or disease progression while also considering the individual brain's current status and unique characteristics. While current deep generative models can produce high-resolution anatomically accurate templates for population-wide studies, their ability to predict future aging trajectories for individuals remains limited, particularly in capturing subject-specific neuroanatomical variations over time. In this study, we introduce Individualized Brain Synthesis (InBrainSyn), a framework for synthesizing high-resolution subject-specific longitudinal MRI scans that simulate neurodegeneration in both Alzheimer's disease (AD) and normal aging. InBrainSyn uses a parallel transport algorithm to adapt the population-level aging trajectories learned by a generative deep template network, enabling individualized aging synthesis. As InBrainSyn uses diffeomorphic transformations to simulate aging, the synthesized images are topologically consistent with the original anatomy by design. We evaluated InBrainSyn both quantitatively and qualitatively on AD and healthy control cohorts from the Open Access Series of Imaging Studies - version 3 dataset. Experimentally, InBrainSyn can also model neuroanatomical transitions between normal aging and AD. An evaluation of an external set supports its generalizability. Overall, with only a single baseline scan, InBrainSyn synthesizes realistic 3D spatiotemporal T1w MRI scans, producing personalized longitudinal aging trajectories. The code for InBrainSyn is available at: https://github.com/Fjr9516/InBrainSyn.
Unsupervised Domain Adaptation for Pediatric Brain Tumor Segmentation
Jun 24, 2024



Abstract:Significant advances have been made toward building accurate automatic segmentation models for adult gliomas. However, the performance of these models often degrades when applied to pediatric glioma due to their imaging and clinical differences (domain shift). Obtaining sufficient annotated data for pediatric glioma is typically difficult because of its rare nature. Also, manual annotations are scarce and expensive. In this work, we propose Domain-Adapted nnU-Net (DA-nnUNet) to perform unsupervised domain adaptation from adult glioma (source domain) to pediatric glioma (target domain). Specifically, we add a domain classifier connected with a gradient reversal layer (GRL) to a backbone nnU-Net. Once the classifier reaches a very high accuracy, the GRL is activated with the goal of transferring domain-invariant features from the classifier to the segmentation model while preserving segmentation accuracy on the source domain. The accuracy of the classifier slowly degrades to chance levels. No annotations are used in the target domain. The method is compared to 8 different supervised models using BraTS-Adult glioma (N=1251) and BraTS-PED glioma data (N=99). The proposed method shows notable performance enhancements in the tumor core (TC) region compared to the model that only uses adult data: ~32% better Dice scores and ~20 better 95th percentile Hausdorff distances. Moreover, our unsupervised approach shows no statistically significant difference compared to the practical upper bound model using manual annotations from both datasets in TC region. The code is shared at https://github.com/Fjr9516/DA_nnUNet.
A deformation-based morphometry framework for disentangling Alzheimer's disease from normal aging using learned normal aging templates
Nov 14, 2023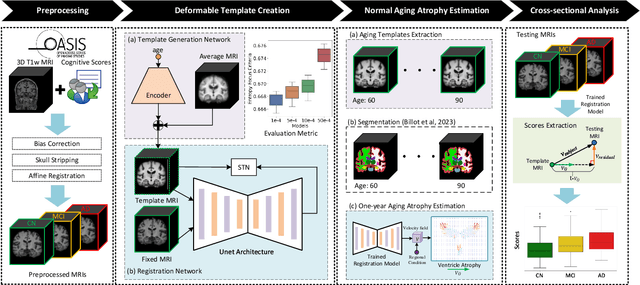
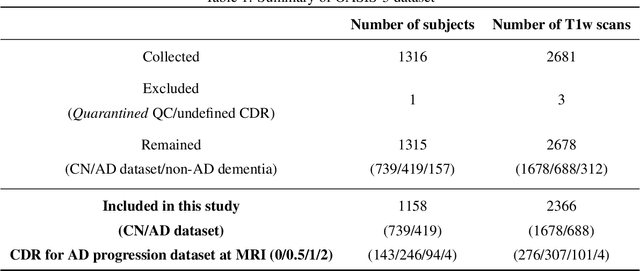
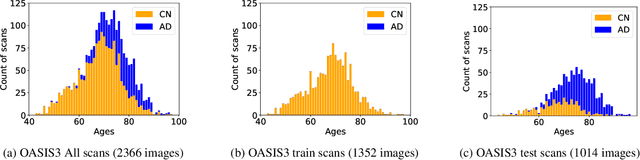
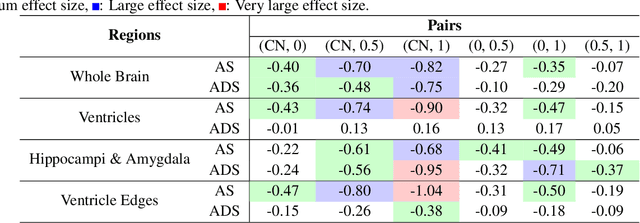
Abstract:Alzheimer's Disease and normal aging are both characterized by brain atrophy. The question of whether AD-related brain atrophy represents accelerated aging or a neurodegeneration process distinct from that in normal aging remains unresolved. Moreover, precisely disentangling AD-related brain atrophy from normal aging in a clinical context is complex. In this study, we propose a deformation-based morphometry framework to estimate normal aging and AD-specific atrophy patterns of subjects from morphological MRI scans. We first leverage deep-learning-based methods to create age-dependent templates of cognitively normal (CN) subjects. These templates model the normal aging atrophy patterns in a CN population. Then, we use the learned diffeomorphic registration to estimate the one-year normal aging pattern at the voxel level. We register the testing image to the 60-year-old CN template in the second step. Finally, normal aging and AD-specific scores are estimated by measuring the alignment of this registration with the one-year normal aging pattern. The methodology was developed and evaluated on the OASIS3 dataset with 1,014 T1-weighted MRI scans. Of these, 326 scans were from CN subjects, and 688 scans were from individuals clinically diagnosed with AD at different stages of clinical severity defined by clinical dementia rating (CDR) scores. The results show that ventricles predominantly follow an accelerated normal aging pattern in subjects with AD. In turn, hippocampi and amygdala regions were affected by both normal aging and AD-specific factors. Interestingly, hippocampi and amygdala regions showed more of an accelerated normal aging pattern for subjects during the early clinical stages of the disease, while the AD-specific score increases in later clinical stages. Our code is freely available at https://github.com/Fjr9516/DBM_with_DL.
Generative Aging of Brain Images with Diffeomorphic Registration
May 31, 2022



Abstract:Analyzing and predicting brain aging is essential for early prognosis and accurate diagnosis of cognitive diseases. The technique of neuroimaging, such as Magnetic Resonance Imaging (MRI), provides a noninvasive means of observing the aging process within the brain. With longitudinal image data collection, data-intensive Artificial Intelligence (AI) algorithms have been used to examine brain aging. However, existing state-of-the-art algorithms tend to be restricted to group-level predictions and suffer from unreal predictions. This paper proposes a methodology for generating longitudinal MRI scans that capture subject-specific neurodegeneration and retain anatomical plausibility in aging. The proposed methodology is developed within the framework of diffeomorphic registration and relies on three key novel technological advances to generate subject-level anatomically plausible predictions: i) a computationally efficient and individualized generative framework based on registration; ii) an aging generative module based on biological linear aging progression; iii) a quality control module to fit registration for generation task. Our methodology was evaluated on 2662 T1-weighted (T1-w) MRI scans from 796 participants from three different cohorts. First, we applied 6 commonly used criteria to demonstrate the aging simulation ability of the proposed methodology; Secondly, we evaluated the quality of the synthetic images using quantitative measurements and qualitative assessment by a neuroradiologist. Overall, the experimental results show that the proposed method can produce anatomically plausible predictions that can be used to enhance longitudinal datasets, in turn enabling data-hungry AI-driven healthcare tools.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge