Jörg Sander
Unsupervised Parameter-free Outlier Detection using HDBSCAN* Outlier Profiles
Nov 13, 2024Abstract:In machine learning and data mining, outliers are data points that significantly differ from the dataset and often introduce irrelevant information that can induce bias in its statistics and models. Therefore, unsupervised methods are crucial to detect outliers if there is limited or no information about them. Global-Local Outlier Scores based on Hierarchies (GLOSH) is an unsupervised outlier detection method within HDBSCAN*, a state-of-the-art hierarchical clustering method. GLOSH estimates outlier scores for each data point by comparing its density to the highest density of the region they reside in the HDBSCAN* hierarchy. GLOSH may be sensitive to HDBSCAN*'s minpts parameter that influences density estimation. With limited knowledge about the data, choosing an appropriate minpts value beforehand is challenging as one or some minpts values may better represent the underlying cluster structure than others. Additionally, in the process of searching for ``potential outliers'', one has to define the number of outliers n a dataset has, which may be impractical and is often unknown. In this paper, we propose an unsupervised strategy to find the ``best'' minpts value, leveraging the range of GLOSH scores across minpts values to identify the value for which GLOSH scores can best identify outliers from the rest of the dataset. Moreover, we propose an unsupervised strategy to estimate a threshold for classifying points into inliers and (potential) outliers without the need to pre-define any value. Our experiments show that our strategies can automatically find the minpts value and threshold that yield the best or near best outlier detection results using GLOSH.
Deep Learning for Automatic Strain Quantification in Arrhythmogenic Right Ventricular Cardiomyopathy
Nov 24, 2023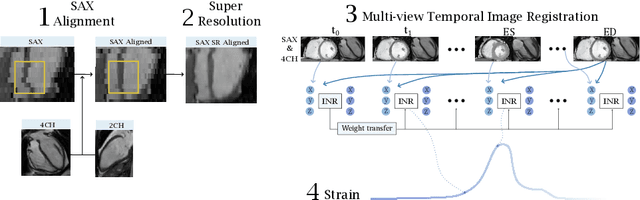
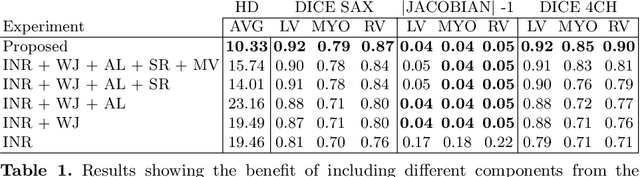
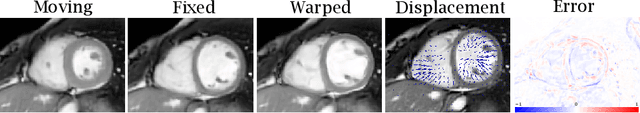
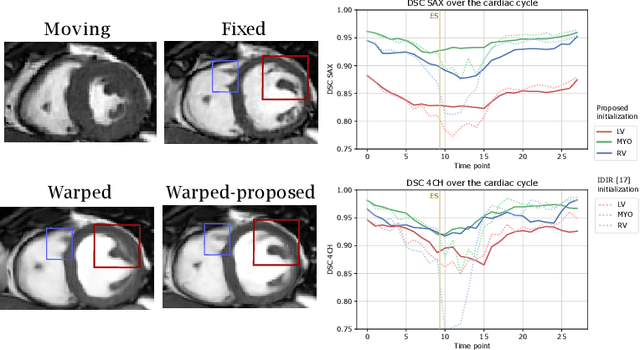
Abstract:Quantification of cardiac motion with cine Cardiac Magnetic Resonance Imaging (CMRI) is an integral part of arrhythmogenic right ventricular cardiomyopathy (ARVC) diagnosis. Yet, the expert evaluation of motion abnormalities with CMRI is a challenging task. To automatically assess cardiac motion, we register CMRIs from different time points of the cardiac cycle using Implicit Neural Representations (INRs) and perform a biomechanically informed regularization inspired by the myocardial incompressibility assumption. To enhance the registration performance, our method first rectifies the inter-slice misalignment inherent to CMRI by performing a rigid registration guided by the long-axis views, and then increases the through-plane resolution using an unsupervised deep learning super-resolution approach. Finally, we propose to synergically combine information from short-axis and 4-chamber long-axis views, along with an initialization to incorporate information from multiple cardiac time points. Thereafter, to quantify cardiac motion, we calculate global and segmental strain over a cardiac cycle and compute the peak strain. The evaluation of the method is performed on a dataset of cine CMRI scans from 47 ARVC patients and 67 controls. Our results show that inter-slice alignment and generation of super-resolved volumes combined with joint analysis of the two cardiac views, notably improves registration performance. Furthermore, the proposed initialization yields more physiologically plausible registrations. The significant differences in the peak strain, discerned between the ARVC patients and healthy controls suggest that automated motion quantification methods may assist in diagnosis and provide further understanding of disease-specific alterations of cardiac motion.
Autoencoding Low-Resolution MRI for Semantically Smooth Interpolation of Anisotropic MRI
Feb 18, 2022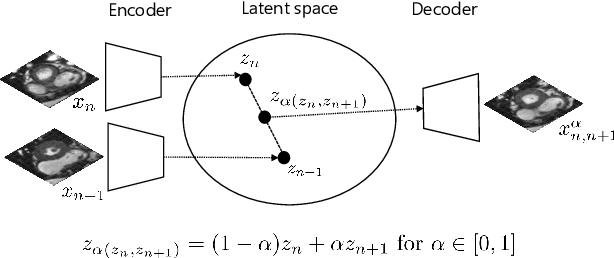
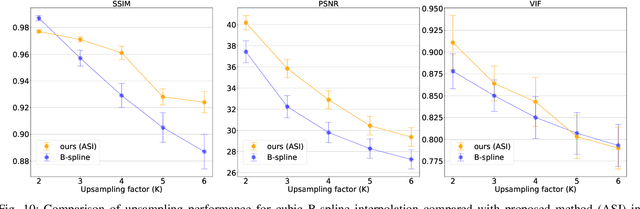
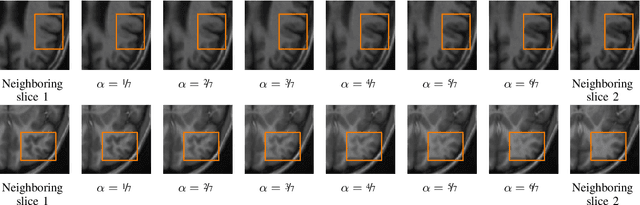
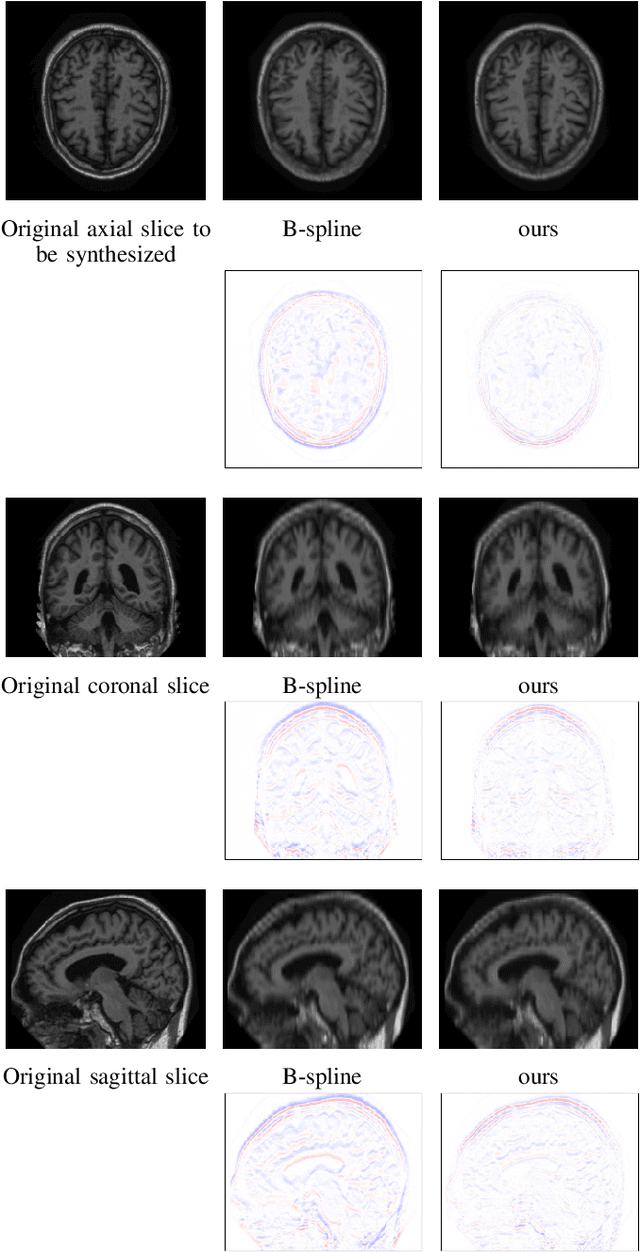
Abstract:High-resolution medical images are beneficial for analysis but their acquisition may not always be feasible. Alternatively, high-resolution images can be created from low-resolution acquisitions using conventional upsampling methods, but such methods cannot exploit high-level contextual information contained in the images. Recently, better performing deep-learning based super-resolution methods have been introduced. However, these methods are limited by their supervised character, i.e. they require high-resolution examples for training. Instead, we propose an unsupervised deep learning semantic interpolation approach that synthesizes new intermediate slices from encoded low-resolution examples. To achieve semantically smooth interpolation in through-plane direction, the method exploits the latent space generated by autoencoders. To generate new intermediate slices, latent space encodings of two spatially adjacent slices are combined using their convex combination. Subsequently, the combined encoding is decoded to an intermediate slice. To constrain the model, a notion of semantic similarity is defined for a given dataset. For this, a new loss is introduced that exploits the spatial relationship between slices of the same volume. During training, an existing in-between slice is generated using a convex combination of its neighboring slice encodings. The method was trained and evaluated using publicly available cardiac cine, neonatal brain and adult brain MRI scans. In all evaluations, the new method produces significantly better results in terms of Structural Similarity Index Measure and Peak Signal-to-Noise Ratio (p< 0.001 using one-sided Wilcoxon signed-rank test) than a cubic B-spline interpolation approach. Given the unsupervised nature of the method, high-resolution training data is not required and hence, the method can be readily applied in clinical settings.
Automatic segmentation with detection of local segmentation failures in cardiac MRI
Nov 13, 2020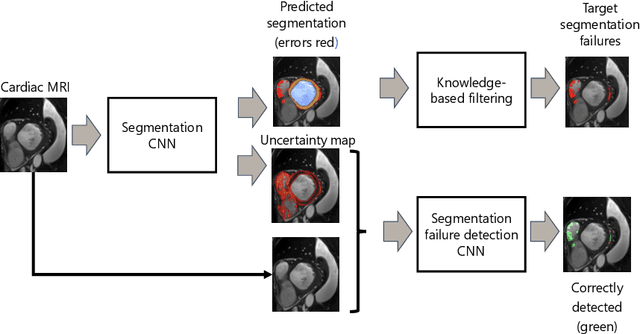
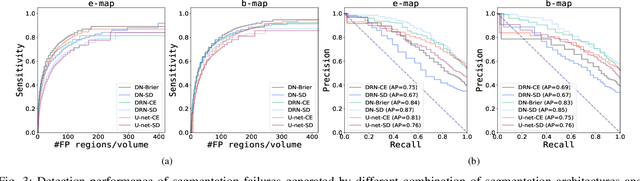
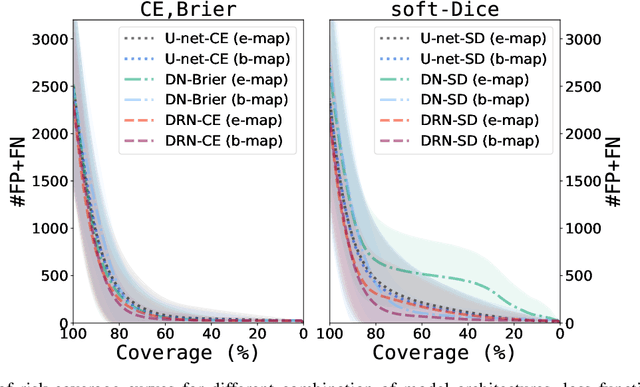
Abstract:Segmentation of cardiac anatomical structures in cardiac magnetic resonance images (CMRI) is a prerequisite for automatic diagnosis and prognosis of cardiovascular diseases. To increase robustness and performance of segmentation methods this study combines automatic segmentation and assessment of segmentation uncertainty in CMRI to detect image regions containing local segmentation failures. Three state-of-the-art convolutional neural networks (CNN) were trained to automatically segment cardiac anatomical structures and obtain two measures of predictive uncertainty: entropy and a measure derived by MC-dropout. Thereafter, using the uncertainties another CNN was trained to detect local segmentation failures that potentially need correction by an expert. Finally, manual correction of the detected regions was simulated. Using publicly available CMR scans from the MICCAI 2017 ACDC challenge, the impact of CNN architecture and loss function for segmentation, and the uncertainty measure was investigated. Performance was evaluated using the Dice coefficient and 3D Hausdorff distance between manual and automatic segmentation. The experiments reveal that combining automatic segmentation with simulated manual correction of detected segmentation failures leads to statistically significant performance increase.
Unsupervised Super-Resolution: Creating High-Resolution Medical Images from Low-Resolution Anisotropic Examples
Oct 25, 2020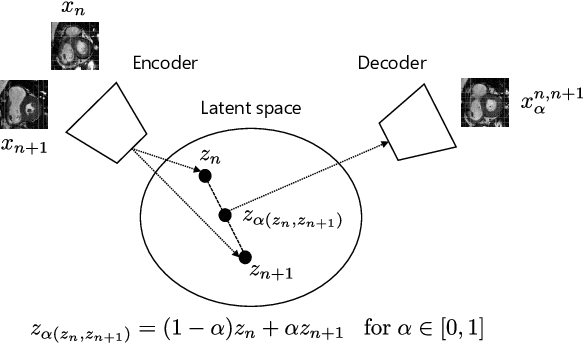
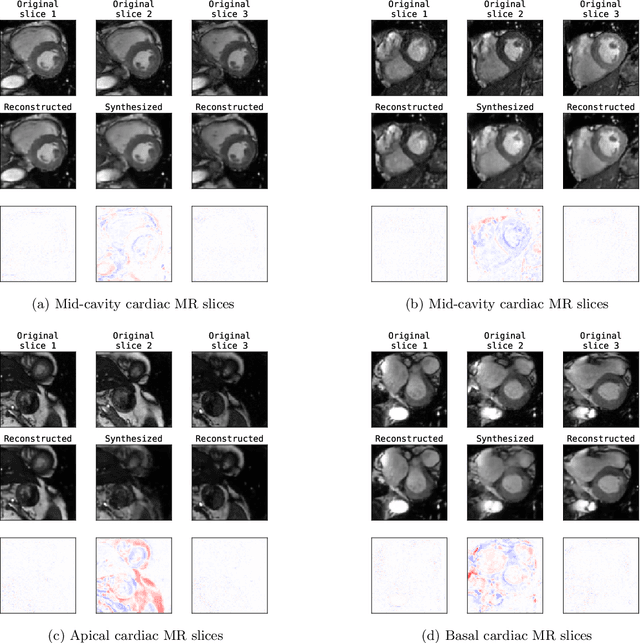
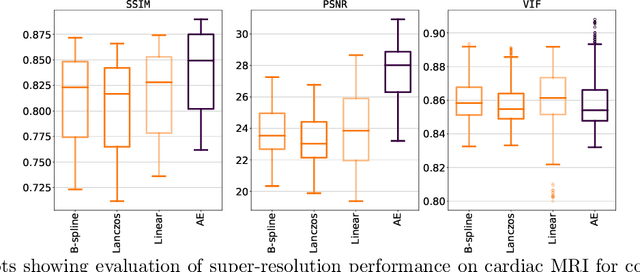
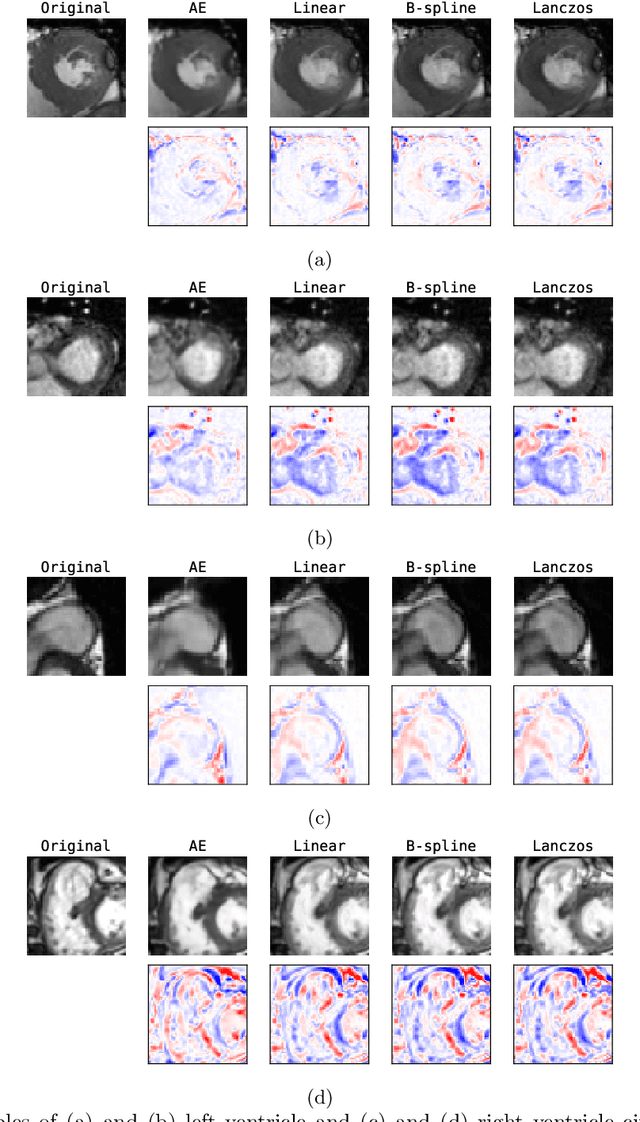
Abstract:Although high resolution isotropic 3D medical images are desired in clinical practice, their acquisition is not always feasible. Instead, lower resolution images are upsampled to higher resolution using conventional interpolation methods. Sophisticated learning-based super-resolution approaches are frequently unavailable in clinical setting, because such methods require training with high-resolution isotropic examples. To address this issue, we propose a learning-based super-resolution approach that can be trained using solely anisotropic images, i.e. without high-resolution ground truth data. The method exploits the latent space, generated by autoencoders trained on anisotropic images, to increase spatial resolution in low-resolution images. The method was trained and evaluated using 100 publicly available cardiac cine MR scans from the Automated Cardiac Diagnosis Challenge (ACDC). The quantitative results show that the proposed method performs better than conventional interpolation methods. Furthermore, the qualitative results indicate that especially finer cardiac structures are synthesized with high quality. The method has the potential to be applied to other anatomies and modalities and can be easily applied to any 3D anisotropic medical image dataset.
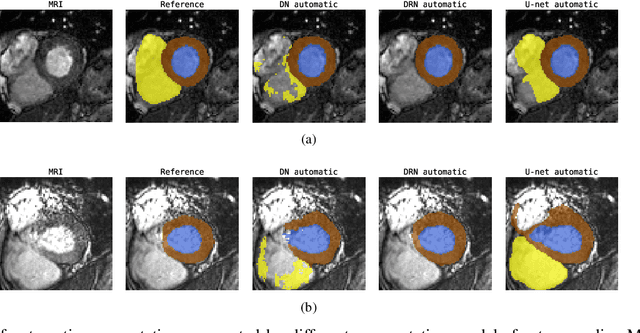
Towards increased trustworthiness of deep learning segmentation methods on cardiac MRI
Sep 29, 2018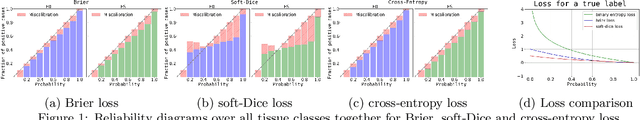
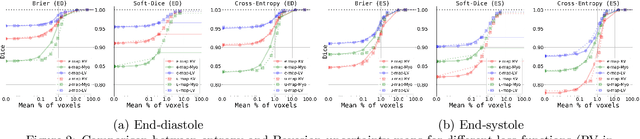
Abstract:Current state-of-the-art deep learning segmentation methods have not yet made a broad entrance into the clinical setting in spite of high demand for such automatic methods. One important reason is the lack of reliability caused by models that fail unnoticed and often locally produce anatomically implausible results that medical experts would not make. This paper presents an automatic image segmentation method based on (Bayesian) dilated convolutional networks (DCNN) that generate segmentation masks and spatial uncertainty maps for the input image at hand. The method was trained and evaluated using segmentation of the left ventricle (LV) cavity, right ventricle (RV) endocardium and myocardium (Myo) at end-diastole (ED) and end-systole (ES) in 100 cardiac 2D MR scans from the MICCAI 2017 Challenge (ACDC). Combining segmentations and uncertainty maps and employing a human-in-the-loop setting, we provide evidence that image areas indicated as highly uncertain regarding the obtained segmentation almost entirely cover regions of incorrect segmentations. The fused information can be harnessed to increase segmentation performance. Our results reveal that we can obtain valuable spatial uncertainty maps with low computational effort using DCNNs.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge