Jérôme Thevenot
Cough-E: A multimodal, privacy-preserving cough detection algorithm for the edge
Oct 31, 2024

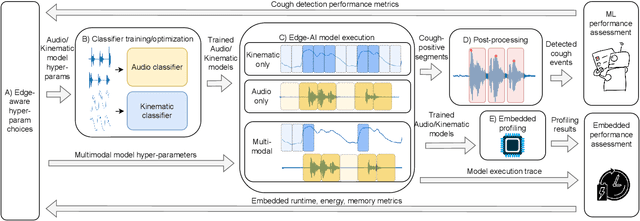

Abstract:Continuous cough monitors can greatly aid doctors in home monitoring and treatment of respiratory diseases. Although many algorithms have been proposed, they still face limitations in data privacy and short-term monitoring. Edge-AI offers a promising solution by processing privacy-sensitive data near the source, but challenges arise in deploying resource-intensive algorithms on constrained devices. From a suitable selection of audio and kinematic signals, our methodology aims at the optimal selection of features via Recursive Feature Elimination with Cross-Validation (RFECV), which exploits the explainability of the selected XGB model. Additionally, it analyzes the use of Mel spectrogram features, instead of the more common MFCC. Moreover, a set of hyperparameters for a multimodal implementation of the classifier is explored. Finally, it evaluates the performance based on clinically relevant event-based metrics. We apply our methodology to develop Cough-E, an energy-efficient, multimodal and edge AI cough detection algorithm. It exploits audio and kinematic data in two distinct classifiers, jointly cooperating for a balanced energy and performance trade-off. We demonstrate that our algorithm can be executed in real-time on an ARM Cortex M33 microcontroller. Cough-E achieves a 70.56\% energy saving when compared to the audio-only approach, at the cost of a 1.26\% relative performance drop, resulting in a 0.78 F1-score. Both Cough-E and the edge-aware model optimization methodology are publicly available as open-source code. This approach demonstrates the benefits of the proposed hardware-aware methodology to enable privacy-preserving cough monitors on the edge, paving the way to efficient cough monitoring.
Multimodal Machine Learning-based Knee Osteoarthritis Progression Prediction from Plain Radiographs and Clinical Data
May 06, 2019
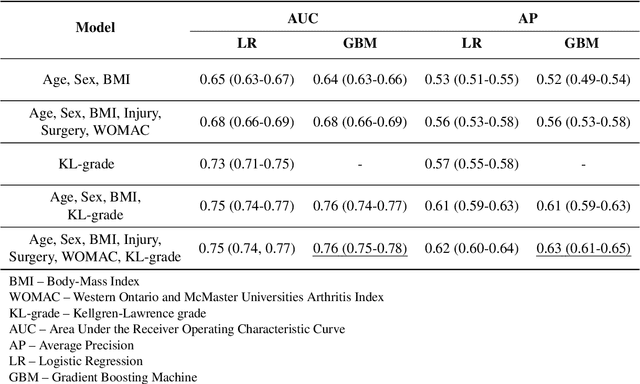


Abstract:Knee osteoarthritis (OA) is the most common musculoskeletal disease without a cure, and current treatment options are limited to symptomatic relief. Prediction of OA progression is a very challenging and timely issue, and it could, if resolved, accelerate the disease modifying drug development and ultimately help to prevent millions of total joint replacement surgeries performed annually. Here, we present a multi-modal machine learning-based OA progression prediction model that utilizes raw radiographic data, clinical examination results and previous medical history of the patient. We validated this approach on an independent test set of 3,918 knee images from 2,129 subjects. Our method yielded area under the ROC curve (AUC) of 0.79 (0.78-0.81) and Average Precision (AP) of 0.68 (0.66-0.70). In contrast, a reference approach, based on logistic regression, yielded AUC of 0.75 (0.74-0.77) and AP of 0.62 (0.60-0.64). The proposed method could significantly improve the subject selection process for OA drug-development trials and help the development of personalized therapeutic plans.
Automatic Knee Osteoarthritis Diagnosis from Plain Radiographs: A Deep Learning-Based Approach
Oct 29, 2017
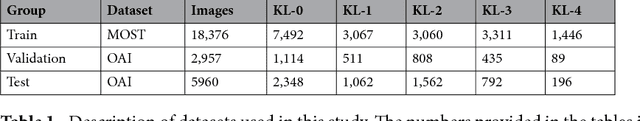


Abstract:Knee osteoarthritis (OA) is the most common musculoskeletal disorder. OA diagnosis is currently conducted by assessing symptoms and evaluating plain radiographs, but this process suffers from subjectivity. In this study, we present a new transparent computer-aided diagnosis method based on the Deep Siamese Convolutional Neural Network to automatically score knee OA severity according to the Kellgren-Lawrence grading scale. We trained our method using the data solely from the Multicenter Osteoarthritis Study and validated it on randomly selected 3,000 subjects (5,960 knees) from Osteoarthritis Initiative dataset. Our method yielded a quadratic Kappa coefficient of 0.83 and average multiclass accuracy of 66.71\% compared to the annotations given by a committee of clinical experts. Here, we also report a radiological OA diagnosis area under the ROC curve of 0.93. We also present attention maps -- given as a class probability distribution -- highlighting the radiological features affecting the network decision. This information makes the decision process transparent for the practitioner, which builds better trust toward automatic methods. We believe that our model is useful for clinical decision making and for OA research; therefore, we openly release our training codes and the data set created in this study.
A novel method for automatic localization of joint area on knee plain radiographs
Apr 05, 2017



Abstract:Osteoarthritis (OA) is a common musculoskeletal condition typically diagnosed from radiographic assessment after clinical examination. However, a visual evaluation made by a practitioner suffers from subjectivity and is highly dependent on the experience. Computer-aided diagnostics (CAD) could improve the objectivity of knee radiographic examination. The first essential step of knee OA CAD is to automatically localize the joint area. However, according to the literature this task itself remains challenging. The aim of this study was to develop novel and computationally efficient method to tackle the issue. Here, three different datasets of knee radiographs were used (n = 473/93/77) to validate the overall performance of the method. Our pipeline consists of two parts: anatomically-based joint area proposal and their evaluation using Histogram of Oriented Gradients and the pre-trained Support Vector Machine classifier scores. The obtained results for the used datasets show the mean intersection over the union equal to: 0.84, 0.79 and 0.78. Using a high-end computer, the method allows to automatically annotate conventional knee radiographs within 14-16ms and high resolution ones within 170ms. Our results demonstrate that the developed method is suitable for large-scale analyses.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge