Irsyad Adam
The Patient is not a Moving Document: A World Model Training Paradigm for Longitudinal EHR
Jan 29, 2026Abstract:Large language models (LLMs) trained with next-word-prediction have achieved success as clinical foundation models. Representations from these language backbones yield strong linear probe performance across biomedical tasks, suggesting that patient semantics emerge from next-token prediction at scale. However, this paradigm treats patients as a document to be summarized rather than a dynamical system to be simulated; a patient's trajectory emerges from their state evolving under interventions and time, requiring models that simulate dynamics rather than predict tokens. To address this, we introduce SMB-Structure, a world model for structured EHR that grounds a joint-embedding prediction architecture (JEPA) with next-token prediction (SFT). SFT grounds our model to reconstruct future patient states in token space, while JEPA predicts those futures in latent space from the initial patient representation alone, forcing trajectory dynamics to be encoded before the next state is observed. We validate across two large-scale cohorts: Memorial Sloan Kettering (23,319 oncology patients; 323,000+ patient-years) and INSPECT (19,402 pulmonary embolism patients). Using a linear probe evaluated at multiple points along the disease trajectory, we demonstrate that our training paradigm learns embeddings that capture disease dynamics not recoverable by autoregressive baselines, enabling SMB-Structure to achieve competitive performance on complex tasks characterized by high patient heterogeneity. Model weights are available at https://huggingface.co/standardmodelbio/SMB-v1-1.7B-Structure.
Platform for Representation and Integration of multimodal Molecular Embeddings
Jul 10, 2025Abstract:Existing machine learning methods for molecular (e.g., gene) embeddings are restricted to specific tasks or data modalities, limiting their effectiveness within narrow domains. As a result, they fail to capture the full breadth of gene functions and interactions across diverse biological contexts. In this study, we have systematically evaluated knowledge representations of biomolecules across multiple dimensions representing a task-agnostic manner spanning three major data sources, including omics experimental data, literature-derived text data, and knowledge graph-based representations. To distinguish between meaningful biological signals from chance correlations, we devised an adjusted variant of Singular Vector Canonical Correlation Analysis (SVCCA) that quantifies signal redundancy and complementarity across different data modalities and sources. These analyses reveal that existing embeddings capture largely non-overlapping molecular signals, highlighting the value of embedding integration. Building on this insight, we propose Platform for Representation and Integration of multimodal Molecular Embeddings (PRISME), a machine learning based workflow using an autoencoder to integrate these heterogeneous embeddings into a unified multimodal representation. We validated this approach across various benchmark tasks, where PRISME demonstrated consistent performance, and outperformed individual embedding methods in missing value imputations. This new framework supports comprehensive modeling of biomolecules, advancing the development of robust, broadly applicable multimodal embeddings optimized for downstream biomedical machine learning applications.
Atherosclerosis through Hierarchical Explainable Neural Network Analysis
Jul 10, 2025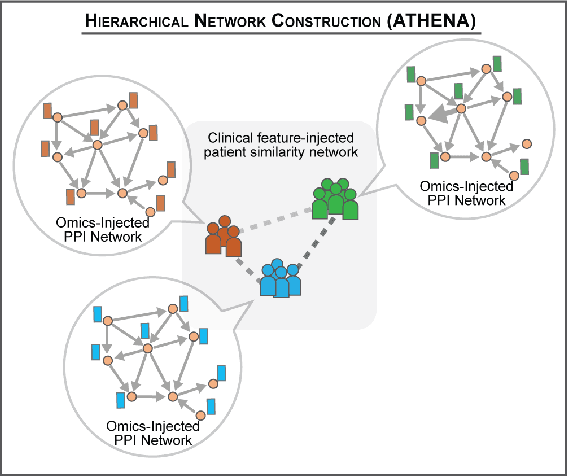
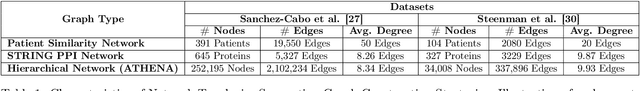
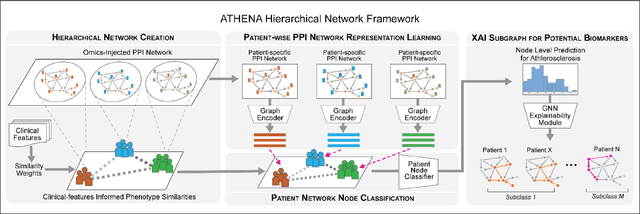
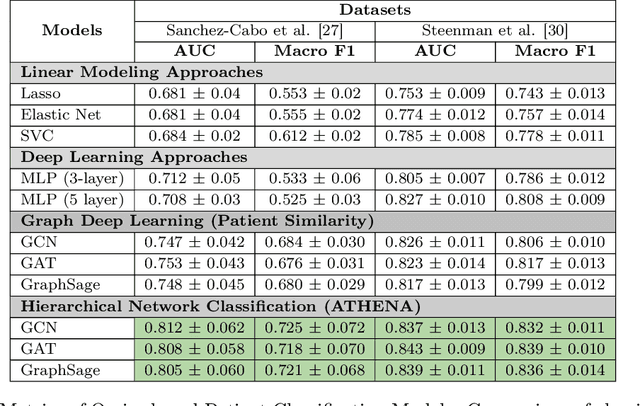
Abstract:In this work, we study the problem pertaining to personalized classification of subclinical atherosclerosis by developing a hierarchical graph neural network framework to leverage two characteristic modalities of a patient: clinical features within the context of the cohort, and molecular data unique to individual patients. Current graph-based methods for disease classification detect patient-specific molecular fingerprints, but lack consistency and comprehension regarding cohort-wide features, which are an essential requirement for understanding pathogenic phenotypes across diverse atherosclerotic trajectories. Furthermore, understanding patient subtypes often considers clinical feature similarity in isolation, without integration of shared pathogenic interdependencies among patients. To address these challenges, we introduce ATHENA: Atherosclerosis Through Hierarchical Explainable Neural Network Analysis, which constructs a novel hierarchical network representation through integrated modality learning; subsequently, it optimizes learned patient-specific molecular fingerprints that reflect individual omics data, enforcing consistency with cohort-wide patterns. With a primary clinical dataset of 391 patients, we demonstrate that this heterogeneous alignment of clinical features with molecular interaction patterns has significantly boosted subclinical atherosclerosis classification performance across various baselines by up to 13% in area under the receiver operating curve (AUC) and 20% in F1 score. Taken together, ATHENA enables mechanistically-informed patient subtype discovery through explainable AI (XAI)-driven subnetwork clustering; this novel integration framework strengthens personalized intervention strategies, thereby improving the prediction of atherosclerotic disease progression and management of their clinical actionable outcomes.
Building an Ethical and Trustworthy Biomedical AI Ecosystem for the Translational and Clinical Integration of Foundational Models
Jul 18, 2024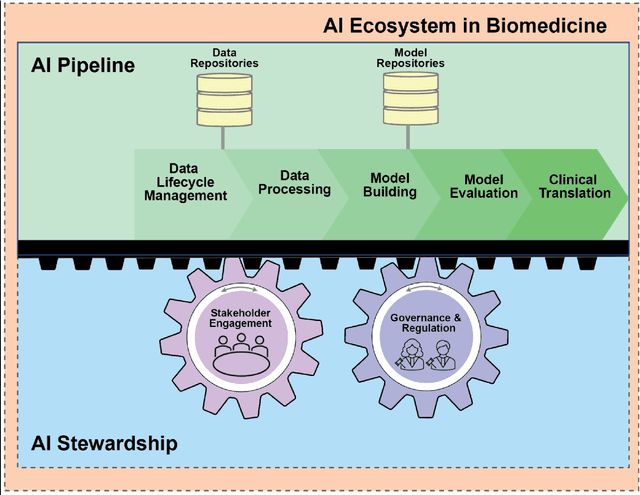
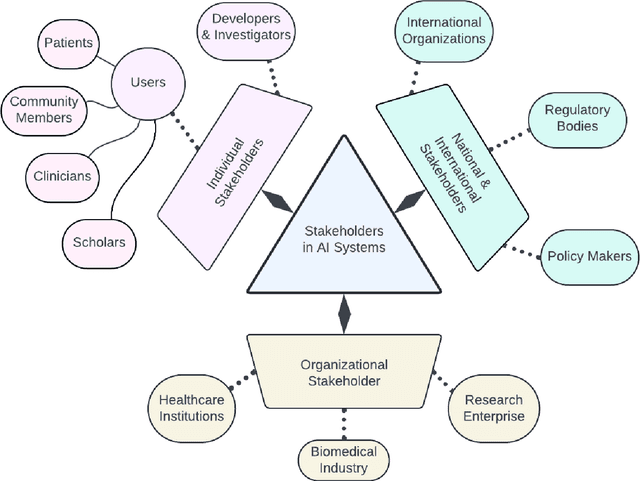
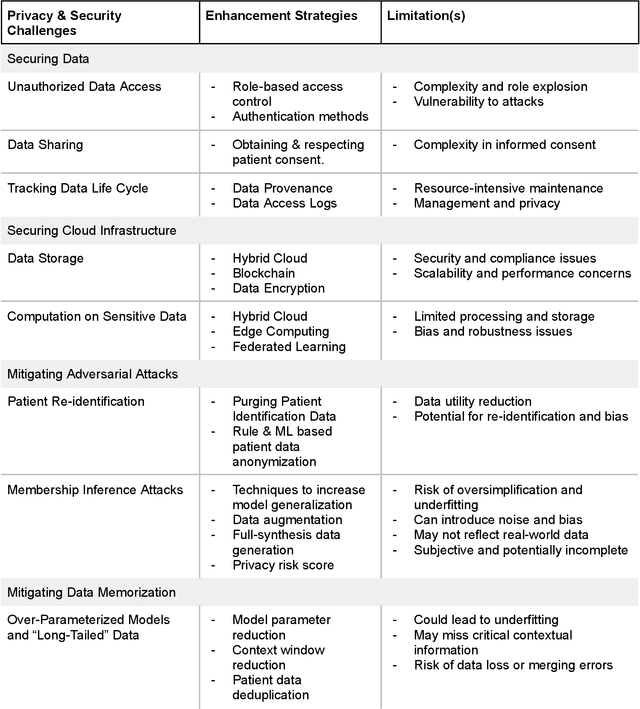
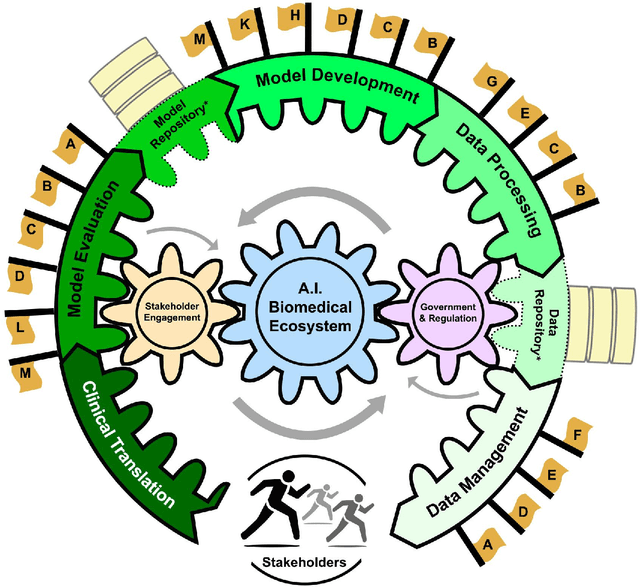
Abstract:Foundational Models (FMs) are emerging as the cornerstone of the biomedical AI ecosystem due to their ability to represent and contextualize multimodal biomedical data. These capabilities allow FMs to be adapted for various tasks, including biomedical reasoning, hypothesis generation, and clinical decision-making. This review paper examines the foundational components of an ethical and trustworthy AI (ETAI) biomedical ecosystem centered on FMs, highlighting key challenges and solutions. The ETAI biomedical ecosystem is defined by seven key components which collectively integrate FMs into clinical settings: Data Lifecycle Management, Data Processing, Model Development, Model Evaluation, Clinical Translation, AI Governance and Regulation, and Stakeholder Engagement. While the potential of biomedical AI is immense, it requires heightened ethical vigilance and responsibility. For instance, biases can arise from data, algorithms, and user interactions, necessitating techniques to assess and mitigate bias prior to, during, and after model development. Moreover, interpretability, explainability, and accountability are key to ensuring the trustworthiness of AI systems, while workflow transparency in training, testing, and evaluation is crucial for reproducibility. Safeguarding patient privacy and security involves addressing challenges in data access, cloud data privacy, patient re-identification, membership inference attacks, and data memorization. Additionally, AI governance and regulation are essential for ethical AI use in biomedicine, guided by global standards. Furthermore, stakeholder engagement is essential at every stage of the AI pipeline and lifecycle for clinical translation. By adhering to these principles, we can harness the transformative potential of AI and develop an ETAI ecosystem.
Explainable Biomedical Hypothesis Generation via Retrieval Augmented Generation enabled Large Language Models
Jul 17, 2024Abstract:The vast amount of biomedical information available today presents a significant challenge for investigators seeking to digest, process, and understand these findings effectively. Large Language Models (LLMs) have emerged as powerful tools to navigate this complex and challenging data landscape. However, LLMs may lead to hallucinatory responses, making Retrieval Augmented Generation (RAG) crucial for achieving accurate information. In this protocol, we present RUGGED (Retrieval Under Graph-Guided Explainable disease Distinction), a comprehensive workflow designed to support investigators with knowledge integration and hypothesis generation, identifying validated paths forward. Relevant biomedical information from publications and knowledge bases are reviewed, integrated, and extracted via text-mining association analysis and explainable graph prediction models on disease nodes, forecasting potential links among drugs and diseases. These analyses, along with biomedical texts, are integrated into a framework that facilitates user-directed mechanism elucidation as well as hypothesis exploration through RAG-enabled LLMs. A clinical use-case demonstrates RUGGED's ability to evaluate and recommend therapeutics for Arrhythmogenic Cardiomyopathy (ACM) and Dilated Cardiomyopathy (DCM), analyzing prescribed drugs for molecular interactions and unexplored uses. The platform minimizes LLM hallucinations, offers actionable insights, and improves the investigation of novel therapeutics.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge