Himeesh Kumar
A deep learning framework for the detection and quantification of drusen and reticular pseudodrusen on optical coherence tomography
Apr 05, 2022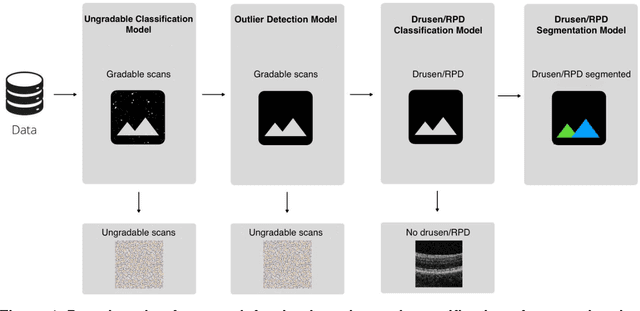
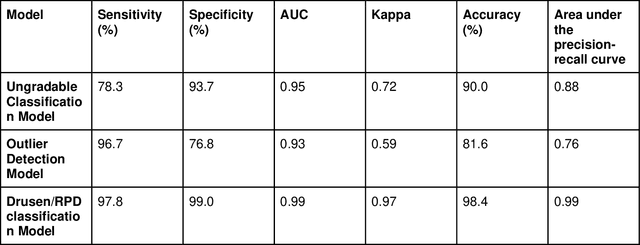
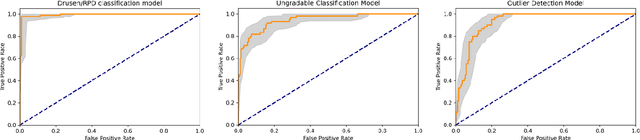
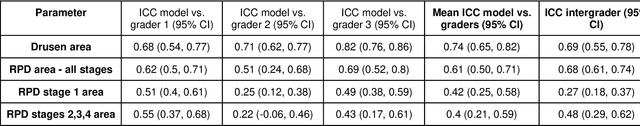
Abstract:Purpose - To develop and validate a deep learning (DL) framework for the detection and quantification of drusen and reticular pseudodrusen (RPD) on optical coherence tomography scans. Design - Development and validation of deep learning models for classification and feature segmentation. Methods - A DL framework was developed consisting of a classification model and an out-of-distribution (OOD) detection model for the identification of ungradable scans; a classification model to identify scans with drusen or RPD; and an image segmentation model to independently segment lesions as RPD or drusen. Data were obtained from 1284 participants in the UK Biobank (UKBB) with a self-reported diagnosis of age-related macular degeneration (AMD) and 250 UKBB controls. Drusen and RPD were manually delineated by five retina specialists. The main outcome measures were sensitivity, specificity, area under the ROC curve (AUC), kappa, accuracy and intraclass correlation coefficient (ICC). Results - The classification models performed strongly at their respective tasks (0.95, 0.93, and 0.99 AUC, respectively, for the ungradable scans classifier, the OOD model, and the drusen and RPD classification model). The mean ICC for drusen and RPD area vs. graders was 0.74 and 0.61, respectively, compared with 0.69 and 0.68 for intergrader agreement. FROC curves showed that the model's sensitivity was close to human performance. Conclusions - The models achieved high classification and segmentation performance, similar to human performance. Application of this robust framework will further our understanding of RPD as a separate entity from drusen in both research and clinical settings.
Which Generative Adversarial Network Yields High-Quality Synthetic Medical Images: Investigation Using AMD Image Datasets
Mar 25, 2022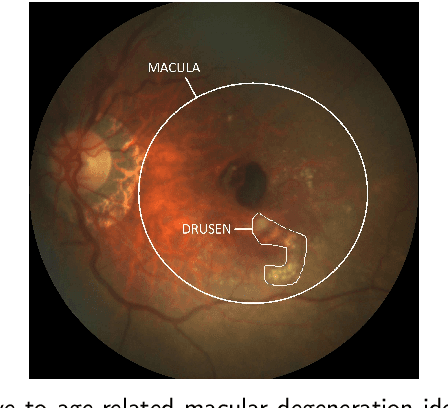
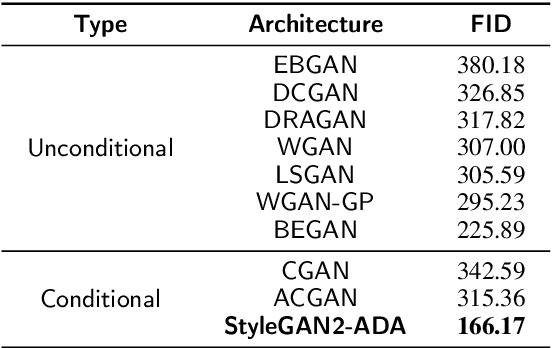

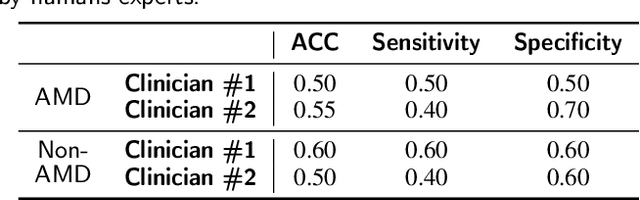
Abstract:Deep learning has been proposed for the assessment and classification of medical images. However, many medical image databases with appropriately labeled and annotated images are small and imbalanced, and thus unsuitable to train and validate such models. The option is to generate synthetic images and one successful technique has been patented which limits its use for others. We have developed a free-access, alternate method for generating synthetic high-resolution images using Generative Adversarial Networks (GAN) for data augmentation and showed their effectiveness using eye-fundus images for Age-Related Macular Degeneration (AMD) identification. Ten different GAN architectures were compared to generate synthetic eye-fundus images with and without AMD. Data from three public databases were evaluated using the Fr\'echet Inception Distance (FID), two clinical experts and deep-learning classification. The results show that StyleGAN2 reached the lowest FID (166.17), and clinicians could not accurately differentiate between real and synthetic images. ResNet-18 architecture obtained the best performance with 85% accuracy and outperformed the two experts in detecting AMD fundus images, whose average accuracy was 77.5%. These results are similar to a recently patented method, and will provide an alternative to generating high-quality synthetic medical images. Free access has been provided to the entire method to facilitate the further development of this field.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge