Harsha Pokkalla
PLUTO: Pathology-Universal Transformer
May 13, 2024



Abstract:Pathology is the study of microscopic inspection of tissue, and a pathology diagnosis is often the medical gold standard to diagnose disease. Pathology images provide a unique challenge for computer-vision-based analysis: a single pathology Whole Slide Image (WSI) is gigapixel-sized and often contains hundreds of thousands to millions of objects of interest across multiple resolutions. In this work, we propose PathoLogy Universal TransfOrmer (PLUTO): a light-weight pathology FM that is pre-trained on a diverse dataset of 195 million image tiles collected from multiple sites and extracts meaningful representations across multiple WSI scales that enable a large variety of downstream pathology tasks. In particular, we design task-specific adaptation heads that utilize PLUTO's output embeddings for tasks which span pathology scales ranging from subcellular to slide-scale, including instance segmentation, tile classification, and slide-level prediction. We compare PLUTO's performance to other state-of-the-art methods on a diverse set of external and internal benchmarks covering multiple biologically relevant tasks, tissue types, resolutions, stains, and scanners. We find that PLUTO matches or outperforms existing task-specific baselines and pathology-specific foundation models, some of which use orders-of-magnitude larger datasets and model sizes when compared to PLUTO. Our findings present a path towards a universal embedding to power pathology image analysis, and motivate further exploration around pathology foundation models in terms of data diversity, architectural improvements, sample efficiency, and practical deployability in real-world applications.
Rethinking Machine Learning Model Evaluation in Pathology
Apr 18, 2022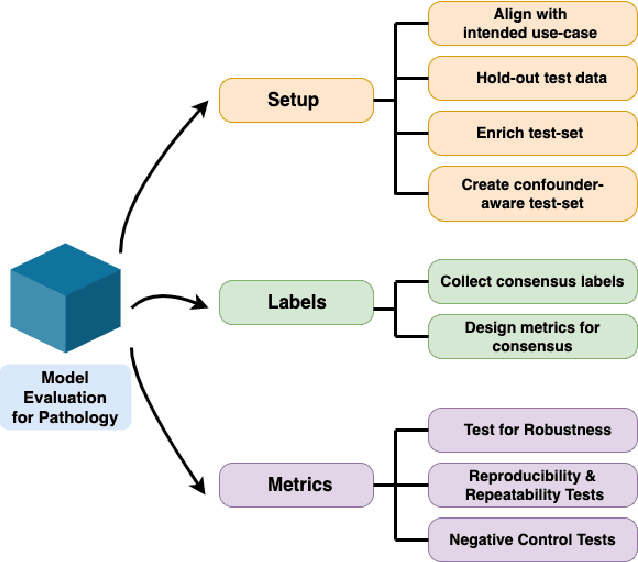
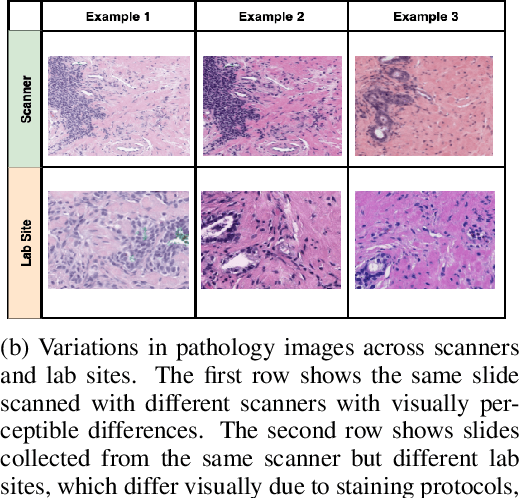
Abstract:Machine Learning has been applied to pathology images in research and clinical practice with promising outcomes. However, standard ML models often lack the rigorous evaluation required for clinical decisions. Machine learning techniques for natural images are ill-equipped to deal with pathology images that are significantly large and noisy, require expensive labeling, are hard to interpret, and are susceptible to spurious correlations. We propose a set of practical guidelines for ML evaluation in pathology that address the above concerns. The paper includes measures for setting up the evaluation framework, effectively dealing with variability in labels, and a recommended suite of tests to address issues related to domain shift, robustness, and confounding variables. We hope that the proposed framework will bridge the gap between ML researchers and domain experts, leading to wider adoption of ML techniques in pathology and improving patient outcomes.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge