Haonan Peng
Efficient Data-driven Joint-level Calibration of Cable-driven Surgical Robots
Aug 02, 2024Abstract:Knowing accurate joint positions is crucial for safe and precise control of laparoscopic surgical robots, especially for the automation of surgical sub-tasks. These robots have often been designed with cable-driven arms and tools because cables allow for larger motors to be placed at the base of the robot, further from the operating area where space is at a premium. However, by connecting the joint to its motor with a cable, any stretch in the cable can lead to errors in kinematic estimation from encoders at the motor, which can result in difficulties for accurate control of the surgical tool. In this work, we propose an efficient data-driven calibration of positioning joints of such robots, in this case the RAVEN-II surgical robotics research platform. While the calibration takes only 8-21 minutes, the accuracy of the calibrated joints remains high during a 6-hour heavily loaded operation, suggesting desirable feasibility in real practice. The calibration models take original robot states as input and are trained using zig-zag trajectories within a desired sparsity, requiring no additional sensors after training. Compared to fixed offset compensation, the Deep Neural Network calibration model can further reduce 76 percent of error and achieve accuracy of 0.104 deg, 0.120 deg, and 0.118 mm in joints 1, 2, and 3, respectively. In contrast to end-to-end models, experiments suggest that the DNN model achieves better accuracy and faster convergence when outputting the error to correct original inaccurate joint positions. Furthermore, a linear regression model is shown to have 160 times faster inference speed than DNN models for application within the 1000 Hz servo control loop, with slightly compromised accuracy.
Ablation Study on Features in Learning-based Joints Calibration of Cable-driven Surgical Robots
Feb 14, 2023



Abstract:With worldwide implementation, millions of surgeries are assisted by surgical robots. The cable-drive mechanism on many surgical robots allows flexible, light, and compact arms and tools. However, the slack and stretch of the cables and the backlash of the gears introduce inevitable errors from motor poses to joint poses, and thus forwarded to the pose and orientation of the end-effector. In this paper, a learning-based calibration using a deep neural network is proposed, which reduces the unloaded pose RMSE of joints 1, 2, 3 to 0.3003 deg, 0.2888 deg, 0.1565 mm, and loaded pose RMSE of joints 1, 2, 3 to 0.4456 deg, 0.3052 deg, 0.1900 mm, respectively. Then, removal ablation and inaccurate ablation are performed to study which features of the DNN model contribute to the calibration accuracy. The results suggest that raw joint poses and motor torques are the most important features. For joint poses, the removal ablation shows that DNN model can derive this information from end-effector pose and orientation. For motor torques, the direction is much more important than amplitude.
Reducing Annotating Load: Active Learning with Synthetic Images in Surgical Instrument Segmentation
Aug 07, 2021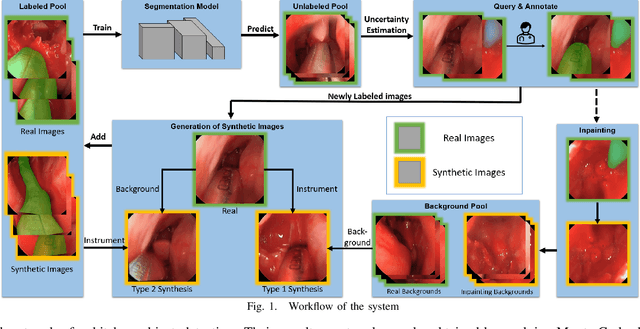
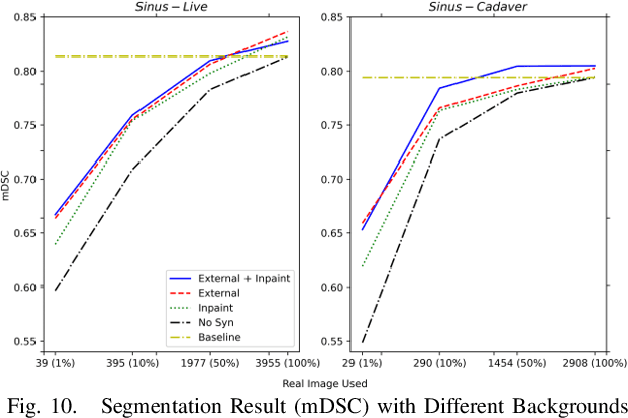
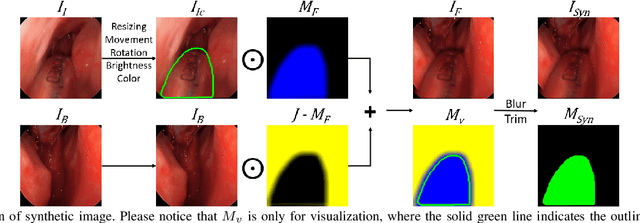
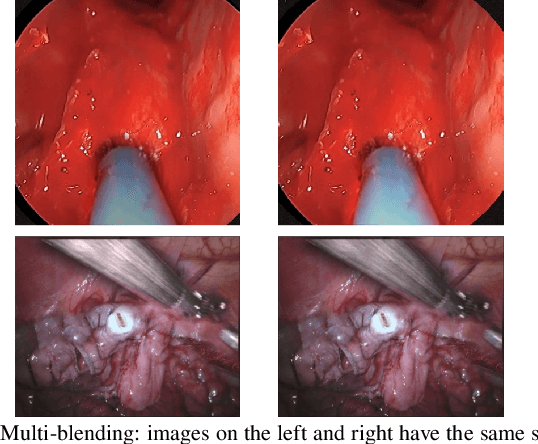
Abstract:Accurate instrument segmentation in endoscopic vision of robot-assisted surgery is challenging due to reflection on the instruments and frequent contacts with tissue. Deep neural networks (DNN) show competitive performance and are in favor in recent years. However, the hunger of DNN for labeled data poses a huge workload of annotation. Motivated by alleviating this workload, we propose a general embeddable method to decrease the usage of labeled real images, using active generated synthetic images. In each active learning iteration, the most informative unlabeled images are first queried by active learning and then labeled. Next, synthetic images are generated based on these selected images. The instruments and backgrounds are cropped out and randomly combined with each other with blending and fusion near the boundary. The effectiveness of the proposed method is validated on 2 sinus surgery datasets and 1 intraabdominal surgery dataset. The results indicate a considerable improvement in performance, especially when the budget for annotation is small. The effectiveness of different types of synthetic images, blending methods, and external background are also studied. All the code is open-sourced at: https://github.com/HaonanPeng/active_syn_generator.
Multi-frame Feature Aggregation for Real-time Instrument Segmentation in Endoscopic Video
Nov 17, 2020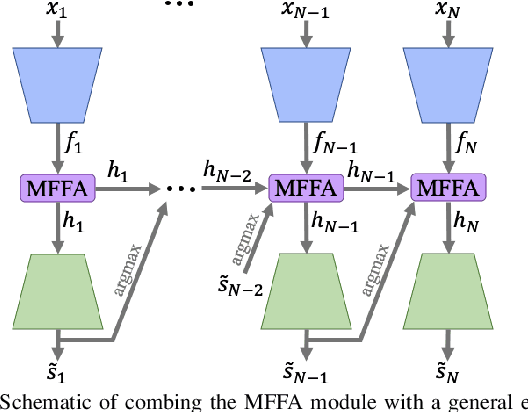
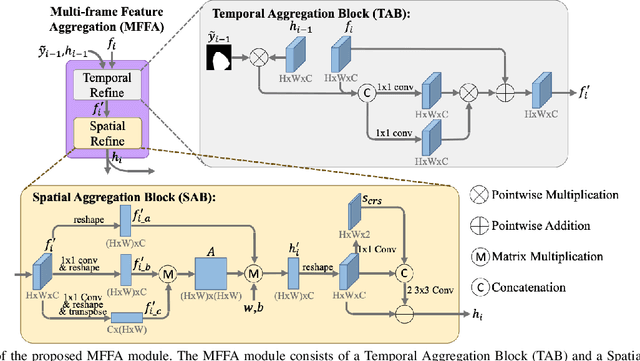
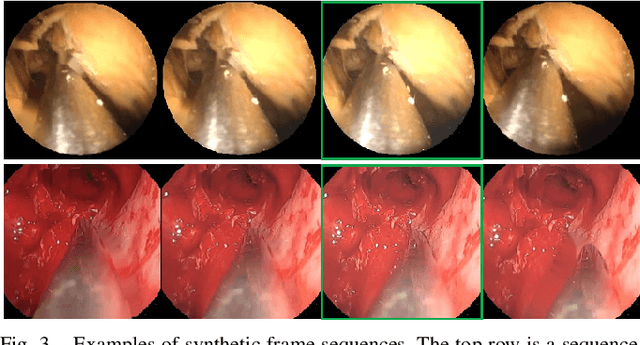
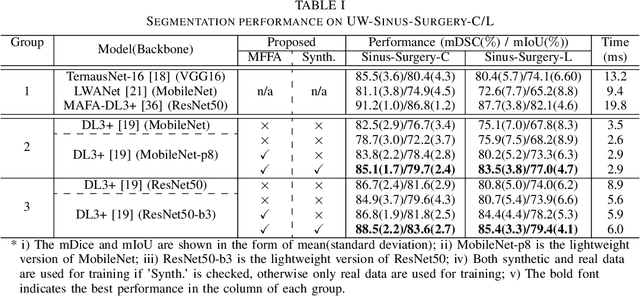
Abstract:Deep learning-based methods have achieved promising results on surgical instrument segmentation. However, the high computation cost may limit the applications of deep models to time-sensitive tasks such as online surgical video analysis for robotic-assisted surgery. Also, current performance may still suffer from challenging conditions in surgical images such as various lighting conditions and the presence of blood. We propose a novel Multi-frame Feature Aggregation (MFFA) module that leverages information of neighboring frames for segmentation while reducing the influence of spatial misalignment between frames. The MFFA module also further aggregates features spatially based on the spatial self-attention mechanism. Neighboring frames usually have similar appearances, so we consider feature aggregation over a frame sequence as an iterative feature aggregation procedure. By distributing the computational workload of deep feature extraction over each frame in a sequence, we can use a lightweight encoder to reduce the computation costs. Moreover, public surgical videos usually are not labeled by frame, so we develop a method that can randomly synthesize a surgical frame sequence from a labeled frame to assist network training. We demonstrate that our approach achieves superior performance to corresponding deeper segmentation models on a public endoscopic sinus surgery dataset.
Real-time Data Driven Precision Estimator for RAVEN-II Surgical Robot End Effector Position
Oct 14, 2019



Abstract:Surgical robots have been introduced to operating rooms over the past few decades due to their high sensitivity, small size, and remote controllability. The cable-driven nature of many surgical robots allows the systems to be dexterous and lightweight, with diameters as low as 5mm. However, due to the slack and stretch of the cables and the backlash of the gears, inevitable uncertainties are brought into the kinematics calculation. Since the reported end effector position of surgical robots like RAVEN-II is directly calculated using the motor encoder measurements and forward kinematics, it may contain relatively large error up to 10mm, whereas semi-autonomous functions being introduced into abdominal surgeries require position inaccuracy of at most 1mm. To resolve the problem, a cost-effective, real-time and data-driven pipeline for robot end effector position precision estimation is proposed and tested on RAVEN-II. Analysis shows an improved end effector position error of around 1mm RMS traversing through the entire robot workspace without high-resolution motion tracker.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge