Haochen Mei
Privacy Inference-Empowered Stealthy Backdoor Attack on Federated Learning under Non-IID Scenarios
Jun 13, 2023



Abstract:Federated learning (FL) naturally faces the problem of data heterogeneity in real-world scenarios, but this is often overlooked by studies on FL security and privacy. On the one hand, the effectiveness of backdoor attacks on FL may drop significantly under non-IID scenarios. On the other hand, malicious clients may steal private data through privacy inference attacks. Therefore, it is necessary to have a comprehensive perspective of data heterogeneity, backdoor, and privacy inference. In this paper, we propose a novel privacy inference-empowered stealthy backdoor attack (PI-SBA) scheme for FL under non-IID scenarios. Firstly, a diverse data reconstruction mechanism based on generative adversarial networks (GANs) is proposed to produce a supplementary dataset, which can improve the attacker's local data distribution and support more sophisticated strategies for backdoor attacks. Based on this, we design a source-specified backdoor learning (SSBL) strategy as a demonstration, allowing the adversary to arbitrarily specify which classes are susceptible to the backdoor trigger. Since the PI-SBA has an independent poisoned data synthesis process, it can be integrated into existing backdoor attacks to improve their effectiveness and stealthiness in non-IID scenarios. Extensive experiments based on MNIST, CIFAR10 and Youtube Aligned Face datasets demonstrate that the proposed PI-SBA scheme is effective in non-IID FL and stealthy against state-of-the-art defense methods.
Automatic Segmentation of Organs-at-Risk from Head-and-Neck CT using Separable Convolutional Neural Network with Hard-Region-Weighted Loss
Feb 03, 2021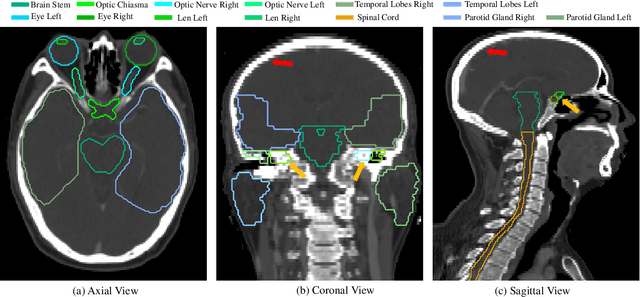
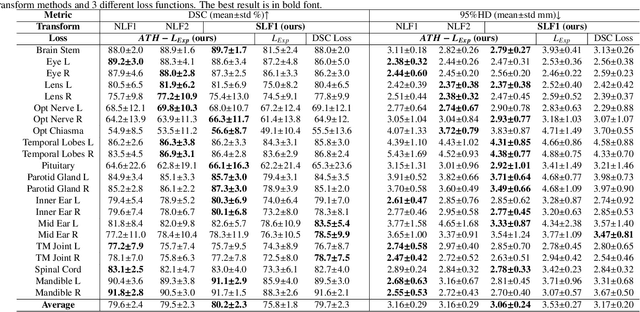
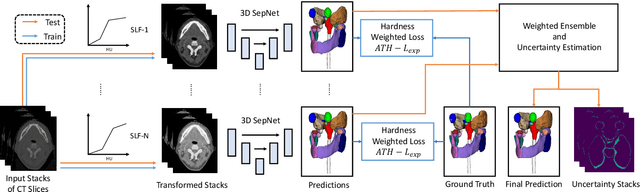
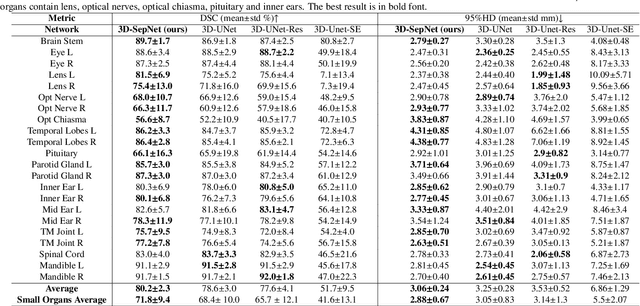
Abstract:Nasopharyngeal Carcinoma (NPC) is a leading form of Head-and-Neck (HAN) cancer in the Arctic, China, Southeast Asia, and the Middle East/North Africa. Accurate segmentation of Organs-at-Risk (OAR) from Computed Tomography (CT) images with uncertainty information is critical for effective planning of radiation therapy for NPC treatment. Despite the stateof-the-art performance achieved by Convolutional Neural Networks (CNNs) for automatic segmentation of OARs, existing methods do not provide uncertainty estimation of the segmentation results for treatment planning, and their accuracy is still limited by several factors, including the low contrast of soft tissues in CT, highly imbalanced sizes of OARs and large inter-slice spacing. To address these problems, we propose a novel framework for accurate OAR segmentation with reliable uncertainty estimation. First, we propose a Segmental Linear Function (SLF) to transform the intensity of CT images to make multiple organs more distinguishable than existing methods based on a simple window width/level that often gives a better visibility of one organ while hiding the others. Second, to deal with the large inter-slice spacing, we introduce a novel 2.5D network (named as 3D-SepNet) specially designed for dealing with clinic HAN CT scans with anisotropic spacing. Thirdly, existing hardness-aware loss function often deal with class-level hardness, but our proposed attention to hard voxels (ATH) uses a voxel-level hardness strategy, which is more suitable to dealing with some hard regions despite that its corresponding class may be easy. Our code is now available at https://github.com/HiLab-git/SepNet.
Automatic Segmentation of Gross Target Volume of Nasopharynx Cancer using Ensemble of Multiscale Deep Neural Networks with Spatial Attention
Jan 27, 2021



Abstract:Radiotherapy is the main treatment modality for nasopharynx cancer. Delineation of Gross Target Volume (GTV) from medical images such as CT and MRI images is a prerequisite for radiotherapy. As manual delineation is time-consuming and laborious, automatic segmentation of GTV has a potential to improve this process. Currently, most of the deep learning-based automatic delineation methods of GTV are mainly performed on medical images like CT images. However, it is challenged by the low contrast between the pathology regions and surrounding soft tissues, small target region, and anisotropic resolution of clinical CT images. To deal with these problems, we propose a 2.5D Convolutional Neural Network (CNN) to handle the difference of inplane and through-plane resolution. Furthermore, we propose a spatial attention module to enable the network to focus on small target, and use channel attention to further improve the segmentation performance. Moreover, we use multi-scale sampling method for training so that the networks can learn features at different scales, which are combined with a multi-model ensemble method to improve the robustness of segmentation results. We also estimate the uncertainty of segmentation results based on our model ensemble, which is of great importance for indicating the reliability of automatic segmentation results for radiotherapy planning.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge