Haobo Zhu
Adversarial Drift-Aware Predictive Transfer: Toward Durable Clinical AI
Jan 21, 2026Abstract:Clinical AI systems frequently suffer performance decay post-deployment due to temporal data shifts, such as evolving populations, diagnostic coding updates (e.g., ICD-9 to ICD-10), and systemic shocks like the COVID-19 pandemic. Addressing this ``aging'' effect via frequent retraining is often impractical due to computational costs and privacy constraints. To overcome these hurdles, we introduce Adversarial Drift-Aware Predictive Transfer (ADAPT), a novel framework designed to confer durability against temporal drift with minimal retraining. ADAPT innovatively constructs an uncertainty set of plausible future models by combining historical source models and limited current data. By optimizing worst-case performance over this set, it balances current accuracy with robustness against degradation due to future drifts. Crucially, ADAPT requires only summary-level model estimators from historical periods, preserving data privacy and ensuring operational simplicity. Validated on longitudinal suicide risk prediction using electronic health records from Mass General Brigham (2005--2021) and Duke University Health Systems, ADAPT demonstrated superior stability across coding transitions and pandemic-induced shifts. By minimizing annual performance decay without labeling or retraining future data, ADAPT offers a scalable pathway for sustaining reliable AI in high-stakes healthcare environments.
Multi-scale, Data-driven and Anatomically Constrained Deep Learning Image Registration for Adult and Fetal Echocardiography
Sep 11, 2023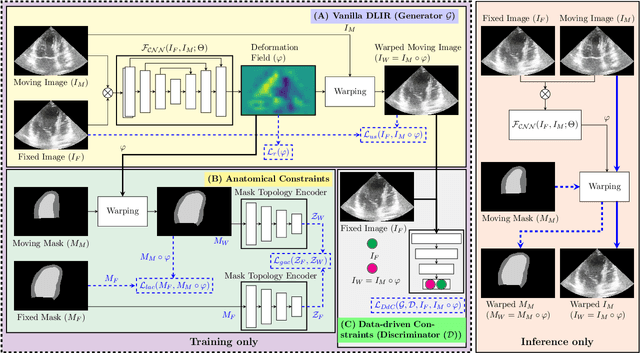

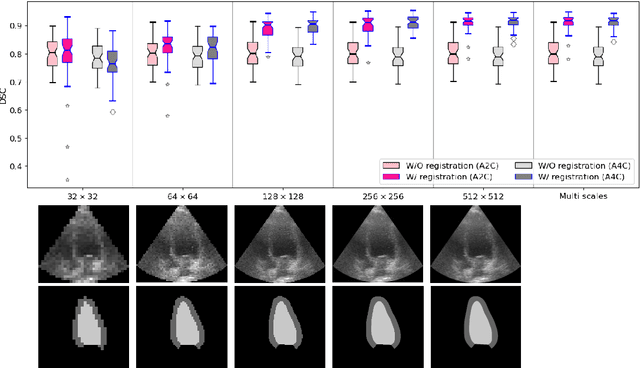
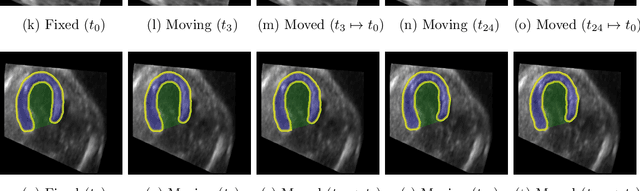
Abstract:Temporal echocardiography image registration is a basis for clinical quantifications such as cardiac motion estimation, myocardial strain assessments, and stroke volume quantifications. In past studies, deep learning image registration (DLIR) has shown promising results and is consistently accurate and precise, requiring less computational time. We propose that a greater focus on the warped moving image's anatomic plausibility and image quality can support robust DLIR performance. Further, past implementations have focused on adult echocardiography, and there is an absence of DLIR implementations for fetal echocardiography. We propose a framework that combines three strategies for DLIR in both fetal and adult echo: (1) an anatomic shape-encoded loss to preserve physiological myocardial and left ventricular anatomical topologies in warped images; (2) a data-driven loss that is trained adversarially to preserve good image texture features in warped images; and (3) a multi-scale training scheme of a data-driven and anatomically constrained algorithm to improve accuracy. Our tests show that good anatomical topology and image textures are strongly linked to shape-encoded and data-driven adversarial losses. They improve different aspects of registration performance in a non-overlapping way, justifying their combination. Despite fundamental distinctions between adult and fetal echo images, we show that these strategies can provide excellent registration results in both adult and fetal echocardiography using the publicly available CAMUS adult echo dataset and our private multi-demographic fetal echo dataset. Our approach outperforms traditional non-DL gold standard registration approaches, including Optical Flow and Elastix. Registration improvements could be translated to more accurate and precise clinical quantification of cardiac ejection fraction, demonstrating a potential for translation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge