Hanna K. Gaggin
Successive Subspace Learning for Cardiac Disease Classification with Two-phase Deformation Fields from Cine MRI
Jan 21, 2023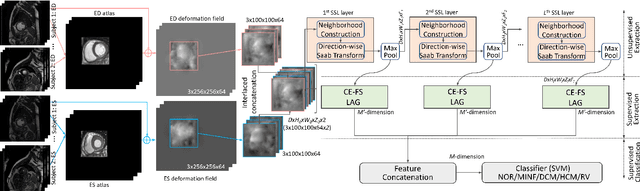
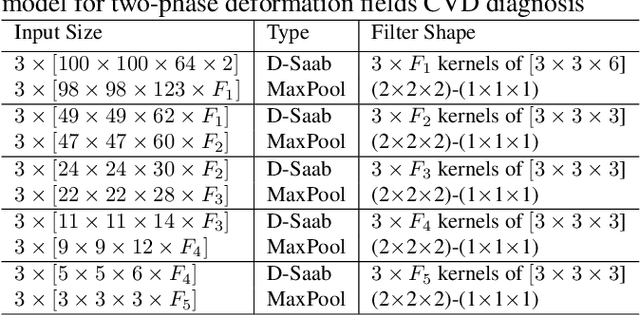
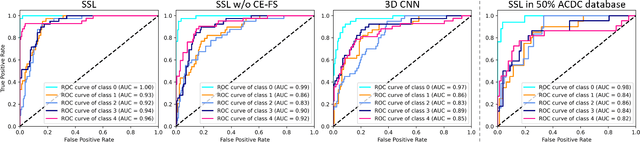
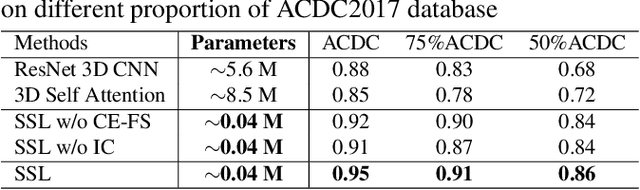
Abstract:Cardiac cine magnetic resonance imaging (MRI) has been used to characterize cardiovascular diseases (CVD), often providing a noninvasive phenotyping tool.~While recently flourished deep learning based approaches using cine MRI yield accurate characterization results, the performance is often degraded by small training samples. In addition, many deep learning models are deemed a ``black box," for which models remain largely elusive in how models yield a prediction and how reliable they are. To alleviate this, this work proposes a lightweight successive subspace learning (SSL) framework for CVD classification, based on an interpretable feedforward design, in conjunction with a cardiac atlas. Specifically, our hierarchical SSL model is based on (i) neighborhood voxel expansion, (ii) unsupervised subspace approximation, (iii) supervised regression, and (iv) multi-level feature integration. In addition, using two-phase 3D deformation fields, including end-diastolic and end-systolic phases, derived between the atlas and individual subjects as input offers objective means of assessing CVD, even with small training samples. We evaluate our framework on the ACDC2017 database, comprising one healthy group and four disease groups. Compared with 3D CNN-based approaches, our framework achieves superior classification performance with 140$\times$ fewer parameters, which supports its potential value in clinical use.
Segmentation of Cardiac Structures via Successive Subspace Learning with Saab Transform from Cine MRI
Jul 22, 2021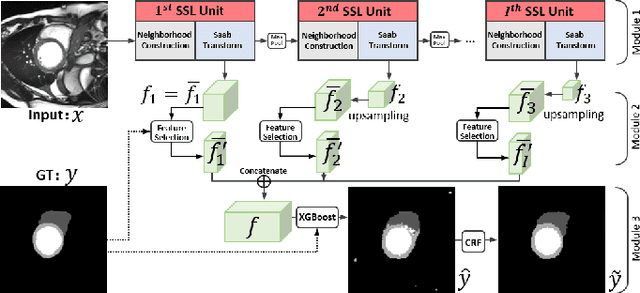
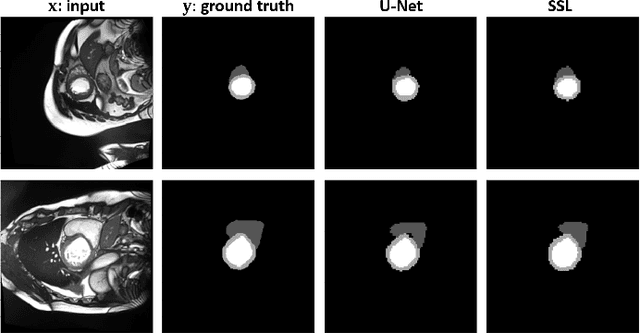
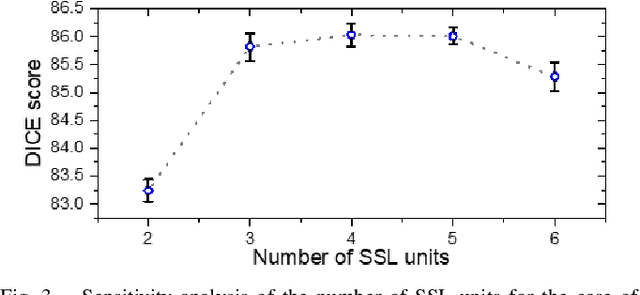
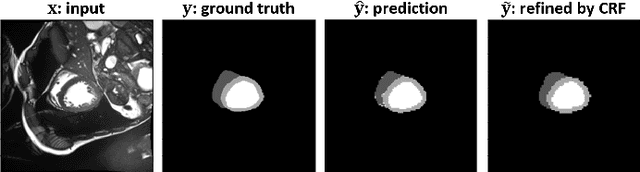
Abstract:Assessment of cardiovascular disease (CVD) with cine magnetic resonance imaging (MRI) has been used to non-invasively evaluate detailed cardiac structure and function. Accurate segmentation of cardiac structures from cine MRI is a crucial step for early diagnosis and prognosis of CVD, and has been greatly improved with convolutional neural networks (CNN). There, however, are a number of limitations identified in CNN models, such as limited interpretability and high complexity, thus limiting their use in clinical practice. In this work, to address the limitations, we propose a lightweight and interpretable machine learning model, successive subspace learning with the subspace approximation with adjusted bias (Saab) transform, for accurate and efficient segmentation from cine MRI. Specifically, our segmentation framework is comprised of the following steps: (1) sequential expansion of near-to-far neighborhood at different resolutions; (2) channel-wise subspace approximation using the Saab transform for unsupervised dimension reduction; (3) class-wise entropy guided feature selection for supervised dimension reduction; (4) concatenation of features and pixel-wise classification with gradient boost; and (5) conditional random field for post-processing. Experimental results on the ACDC 2017 segmentation database, showed that our framework performed better than state-of-the-art U-Net models with 200$\times$ fewer parameters in delineating the left ventricle, right ventricle, and myocardium, thus showing its potential to be used in clinical practice.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge