Franz MJ Pfister
RAISE -- Radiology AI Safety, an End-to-end lifecycle approach
Nov 24, 2023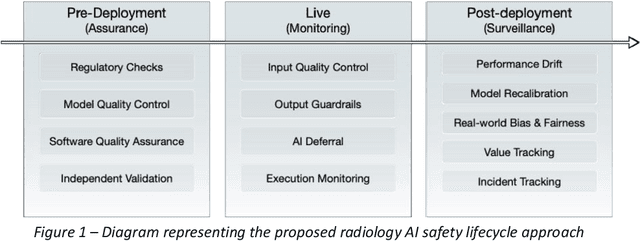
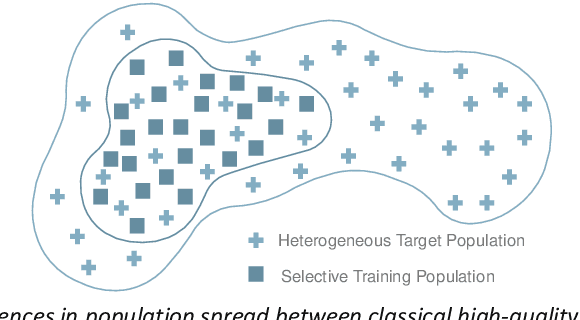
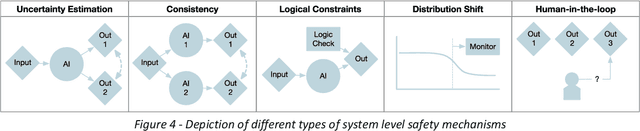
Abstract:The integration of AI into radiology introduces opportunities for improved clinical care provision and efficiency but it demands a meticulous approach to mitigate potential risks as with any other new technology. Beginning with rigorous pre-deployment evaluation and validation, the focus should be on ensuring models meet the highest standards of safety, effectiveness and efficacy for their intended applications. Input and output guardrails implemented during production usage act as an additional layer of protection, identifying and addressing individual failures as they occur. Continuous post-deployment monitoring allows for tracking population-level performance (data drift), fairness, and value delivery over time. Scheduling reviews of post-deployment model performance and educating radiologists about new algorithmic-driven findings is critical for AI to be effective in clinical practice. Recognizing that no single AI solution can provide absolute assurance even when limited to its intended use, the synergistic application of quality assurance at multiple levels - regulatory, clinical, technical, and ethical - is emphasized. Collaborative efforts between stakeholders spanning healthcare systems, industry, academia, and government are imperative to address the multifaceted challenges involved. Trust in AI is an earned privilege, contingent on a broad set of goals, among them transparently demonstrating that the AI adheres to the same rigorous safety, effectiveness and efficacy standards as other established medical technologies. By doing so, developers can instil confidence among providers and patients alike, enabling the responsible scaling of AI and the realization of its potential benefits. The roadmap presented herein aims to expedite the achievement of deployable, reliable, and safe AI in radiology.
Wearable-based Parkinson's Disease Severity Monitoring using Deep Learning
Apr 24, 2019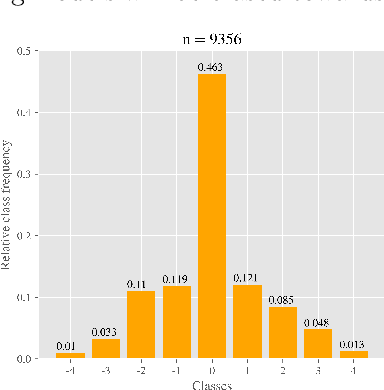
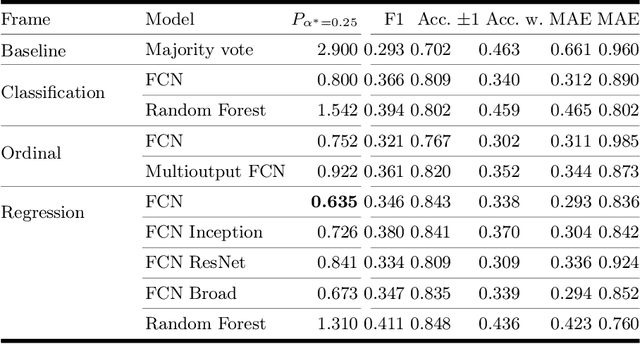
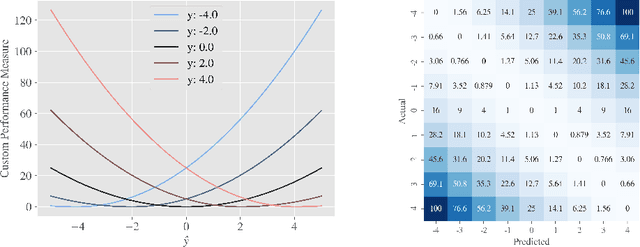
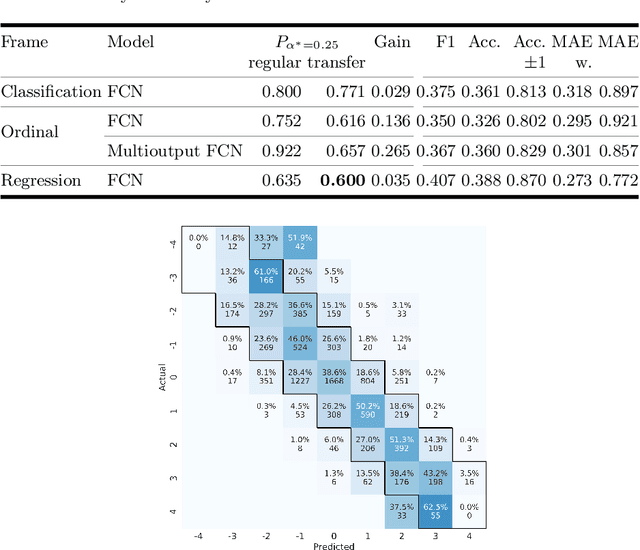
Abstract:One major challenge in the medication of Parkinson's disease is that the severity of the disease, reflected in the patients' motor state, cannot be measured using accessible biomarkers. Therefore, we develop and examine a variety of statistical models to detect the motor state of such patients based on sensor data from a wearable device. We find that deep learning models consistently outperform a classical machine learning model applied on hand-crafted features in this time series classification task. Furthermore, our results suggest that treating this problem as a regression instead of an ordinal regression or a classification task is most appropriate. For consistent model evaluation and training, we adopt the leave-one-subject-out validation scheme to the training of deep learning models. We also employ a class-weighting scheme to successfully mitigate the problem of high multi-class imbalances in this domain. In addition, we propose a customized performance measure that reflects the requirements of the involved medical staff on the model. To solve the problem of limited availability of high quality training data, we propose a transfer learning technique which helps to improve model performance substantially. Our results suggest that deep learning techniques offer a high potential to autonomously detect motor states of patients with Parkinson's disease.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge