Derrek Paul Hibar
Large-scale Feature Selection of Risk Genetic Factors for Alzheimer's Disease via Distributed Group Lasso Regression
Apr 27, 2017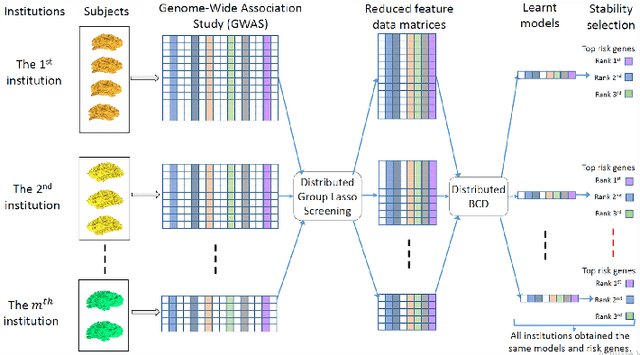
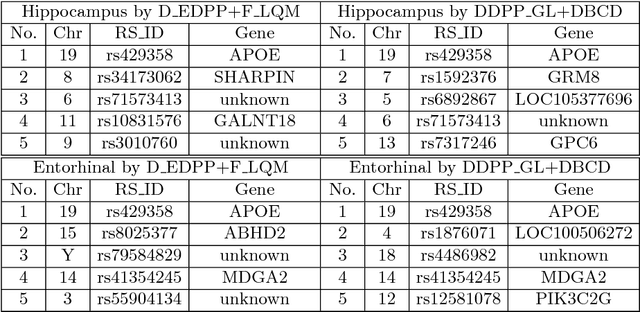
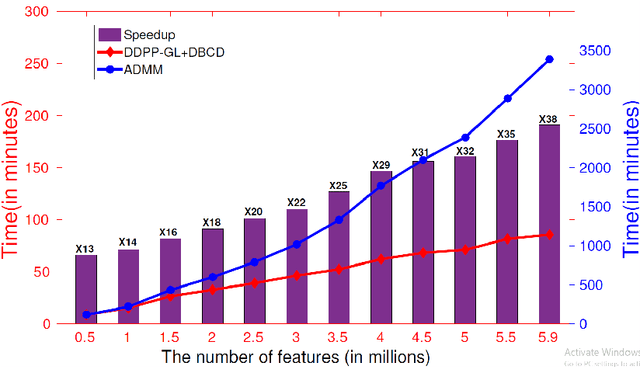
Abstract:Genome-wide association studies (GWAS) have achieved great success in the genetic study of Alzheimer's disease (AD). Collaborative imaging genetics studies across different research institutions show the effectiveness of detecting genetic risk factors. However, the high dimensionality of GWAS data poses significant challenges in detecting risk SNPs for AD. Selecting relevant features is crucial in predicting the response variable. In this study, we propose a novel Distributed Feature Selection Framework (DFSF) to conduct the large-scale imaging genetics studies across multiple institutions. To speed up the learning process, we propose a family of distributed group Lasso screening rules to identify irrelevant features and remove them from the optimization. Then we select the relevant group features by performing the group Lasso feature selection process in a sequence of parameters. Finally, we employ the stability selection to rank the top risk SNPs that might help detect the early stage of AD. To the best of our knowledge, this is the first distributed feature selection model integrated with group Lasso feature selection as well as detecting the risk genetic factors across multiple research institutions system. Empirical studies are conducted on 809 subjects with 5.9 million SNPs which are distributed across several individual institutions, demonstrating the efficiency and effectiveness of the proposed method.
Large-scale Collaborative Imaging Genetics Studies of Risk Genetic Factors for Alzheimer's Disease Across Multiple Institutions
Aug 19, 2016


Abstract:Genome-wide association studies (GWAS) offer new opportunities to identify genetic risk factors for Alzheimer's disease (AD). Recently, collaborative efforts across different institutions emerged that enhance the power of many existing techniques on individual institution data. However, a major barrier to collaborative studies of GWAS is that many institutions need to preserve individual data privacy. To address this challenge, we propose a novel distributed framework, termed Local Query Model (LQM) to detect risk SNPs for AD across multiple research institutions. To accelerate the learning process, we propose a Distributed Enhanced Dual Polytope Projection (D-EDPP) screening rule to identify irrelevant features and remove them from the optimization. To the best of our knowledge, this is the first successful run of the computationally intensive model selection procedure to learn a consistent model across different institutions without compromising their privacy while ranking the SNPs that may collectively affect AD. Empirical studies are conducted on 809 subjects with 5.9 million SNP features which are distributed across three individual institutions. D-EDPP achieved a 66-fold speed-up by effectively identifying irrelevant features.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge