David Wolk
Surface-based parcellation and vertex-wise analysis of ultra high-resolution ex vivo 7 tesla MRI in neurodegenerative diseases
Mar 28, 2024



Abstract:Magnetic resonance imaging (MRI) is the standard modality to understand human brain structure and function in vivo (antemortem). Decades of research in human neuroimaging has led to the widespread development of methods and tools to provide automated volume-based segmentations and surface-based parcellations which help localize brain functions to specialized anatomical regions. Recently ex vivo (postmortem) imaging of the brain has opened-up avenues to study brain structure at sub-millimeter ultra high-resolution revealing details not possible to observe with in vivo MRI. Unfortunately, there has been limited methodological development in ex vivo MRI primarily due to lack of datasets and limited centers with such imaging resources. Therefore, in this work, we present one-of-its-kind dataset of 82 ex vivo T2w whole brain hemispheres MRI at 0.3 mm isotropic resolution spanning Alzheimer's disease and related dementias. We adapted and developed a fast and easy-to-use automated surface-based pipeline to parcellate, for the first time, ultra high-resolution ex vivo brain tissue at the native subject space resolution using the Desikan-Killiany-Tourville (DKT) brain atlas. This allows us to perform vertex-wise analysis in the template space and thereby link morphometry measures with pathology measurements derived from histology. We will open-source our dataset docker container, Jupyter notebooks for ready-to-use out-of-the-box set of tools and command line options to advance ex vivo MRI clinical brain imaging research on the project webpage.
Disentangling brain heterogeneity via semi-supervised deep-learning and MRI: dimensional representations of Alzheimer's Disease
Feb 24, 2021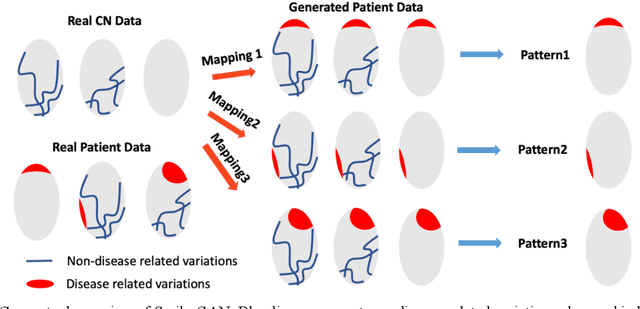
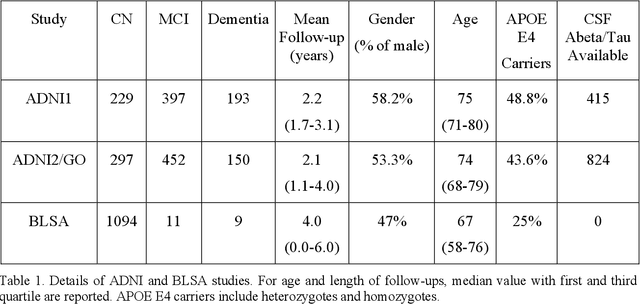
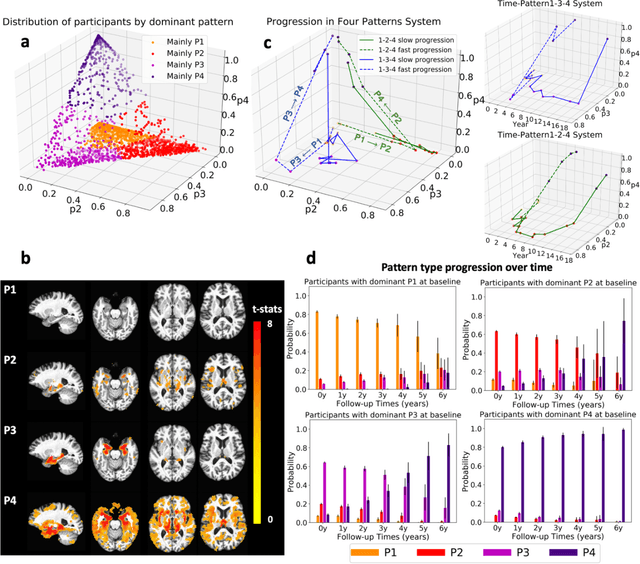
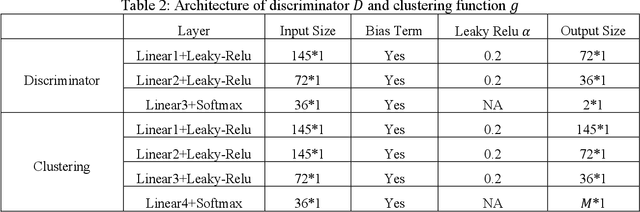
Abstract:Heterogeneity of brain diseases is a challenge for precision diagnosis/prognosis. We describe and validate Smile-GAN (SeMI-supervised cLustEring-Generative Adversarial Network), a novel semi-supervised deep-clustering method, which dissects neuroanatomical heterogeneity, enabling identification of disease subtypes via their imaging signatures relative to controls. When applied to MRIs (2 studies; 2,832 participants; 8,146 scans) including cognitively normal individuals and those with cognitive impairment and dementia, Smile-GAN identified 4 neurodegenerative patterns/axes: P1, normal anatomy and highest cognitive performance; P2, mild/diffuse atrophy and more prominent executive dysfunction; P3, focal medial temporal atrophy and relatively greater memory impairment; P4, advanced neurodegeneration. Further application to longitudinal data revealed two distinct progression pathways: P1$\rightarrow$P2$\rightarrow$P4 and P1$\rightarrow$P3$\rightarrow$P4. Baseline expression of these patterns predicted the pathway and rate of future neurodegeneration. Pattern expression offered better yet complementary performance in predicting clinical progression, compared to amyloid/tau. These deep-learning derived biomarkers offer promise for precision diagnostics and targeted clinical trial recruitment.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge