David Mayerich
GPU-Accelerated RSF Level Set Evolution for Large-Scale Microvascular Segmentation
Apr 03, 2024



Abstract:Microvascular networks are challenging to model because these structures are currently near the diffraction limit for most advanced three-dimensional imaging modalities, including confocal and light sheet microscopy. This makes semantic segmentation difficult, because individual components of these networks fluctuate within the confines of individual pixels. Level set methods are ideally suited to solve this problem by providing surface and topological constraints on the resulting model, however these active contour techniques are extremely time intensive and impractical for terabyte-scale images. We propose a reformulation and implementation of the region-scalable fitting (RSF) level set model that makes it amenable to three-dimensional evaluation using both single-instruction multiple data (SIMD) and single-program multiple-data (SPMD) parallel processing. This enables evaluation of the level set equation on independent regions of the data set using graphics processing units (GPUs), making large-scale segmentation of high-resolution networks practical and inexpensive. We tested this 3D parallel RSF approach on multiple data sets acquired using state-of-the-art imaging techniques to acquire microvascular data, including micro-CT, light sheet fluorescence microscopy (LSFM) and milling microscopy. To assess the performance and accuracy of the RSF model, we conducted a Monte-Carlo-based validation technique to compare results to other segmentation methods. We also provide a rigorous profiling to show the gains in processing speed leveraging parallel hardware. This study showcases the practical application of the RSF model, emphasizing its utility in the challenging domain of segmenting large-scale high-topology network structures with a particular focus on building microvascular models.
Rapid hyperspectral photothermal mid-infrared spectroscopic imaging from sparse data for gynecologic cancer tissue subtyping
Feb 28, 2024



Abstract:Ovarian cancer detection has traditionally relied on a multi-step process that includes biopsy, tissue staining, and morphological analysis by experienced pathologists. While widely practiced, this conventional approach suffers from several drawbacks: it is qualitative, time-intensive, and heavily dependent on the quality of staining. Mid-infrared (MIR) hyperspectral photothermal imaging is a label-free, biochemically quantitative technology that, when combined with machine learning algorithms, can eliminate the need for staining and provide quantitative results comparable to traditional histology. However, this technology is slow. This work presents a novel approach to MIR photothermal imaging that enhances its speed by an order of magnitude. Our method significantly accelerates data collection by capturing a combination of high-resolution and interleaved, lower-resolution infrared band images and applying computational techniques for data interpolation. We effectively minimize data collection requirements by leveraging sparse data acquisition and employing curvelet-based reconstruction algorithms. This method enables the reconstruction of high-quality, high-resolution images from undersampled datasets and achieving a 10X improvement in data acquisition time. We assessed the performance of our sparse imaging methodology using a variety of quantitative metrics, including mean squared error (MSE), structural similarity index (SSIM), and tissue subtype classification accuracies, employing both random forest and convolutional neural network (CNN) models, accompanied by ROC curves. Our statistically robust analysis, based on data from 100 ovarian cancer patient samples and over 65 million data points, demonstrates the method's capability to produce superior image quality and accurately distinguish between different gynecological tissue types with segmentation accuracy exceeding 95%.
Leveraging high-resolution spatial features in mid-infrared spectroscopic imaging to classify tissue subtypes in ovarian cancer
May 19, 2022

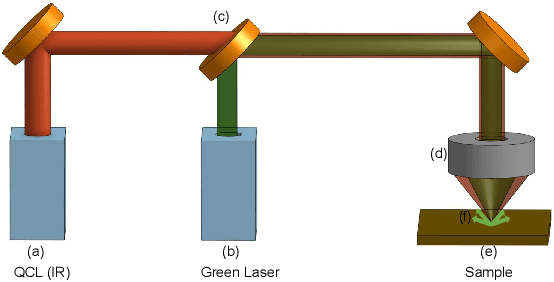

Abstract:Mid-infrared spectroscopic imaging (MIRSI) is an emerging class of label-free techniques being leveraged for digital histopathology. Optical photothermal infrared (O-PTIR) is based on vibrational absorbance imaging using a pump-probe architecture capable of a 10x enhancement in spatial resolution relative to FTIR imaging. This allows truly sub-cellular spectroscopic investigation of tissue at biochemically important fingerprint wavelengths. Modern histopathologic identification of ovarian cancer involves tissue staining followed by morphological pattern recognition. This process is time-consuming, subjective, and requires extensive expertise. In this paper, we present the first label-free automated histological classification of ovarian tissue sub-types using MIRSI. We demonstrate that enhanced resolution of sub-cellular features, combined with spectroscopic information, enables reliable classification (0.98 AUC) of ovarian cell sub-types. Moreover, we present statistically robust validation from 74 patient samples with over 60 million data points. This demonstrates that sub-cellular resolution from five wavenumbers is sufficient to outperform state-of-the-art diffraction-limited techniques from up to 374 different wavenumbers. O-PTIR also performs measurements in back-reflection geometry, opening the door to future in vivo studies on glass slides.
Hyperspectral-Multispectral Image Fusion with Weighted LASSO
Mar 15, 2020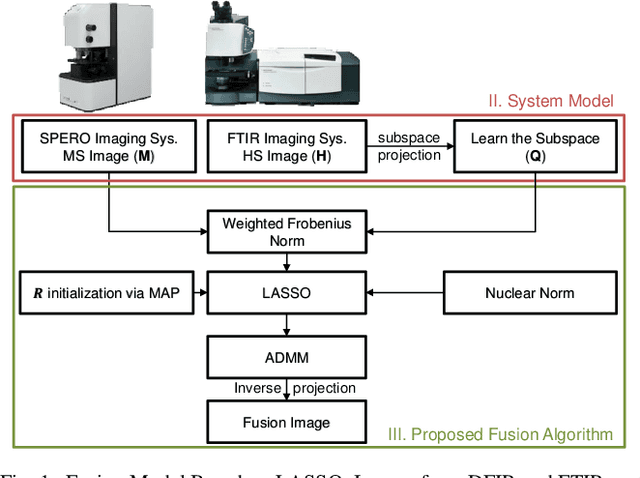
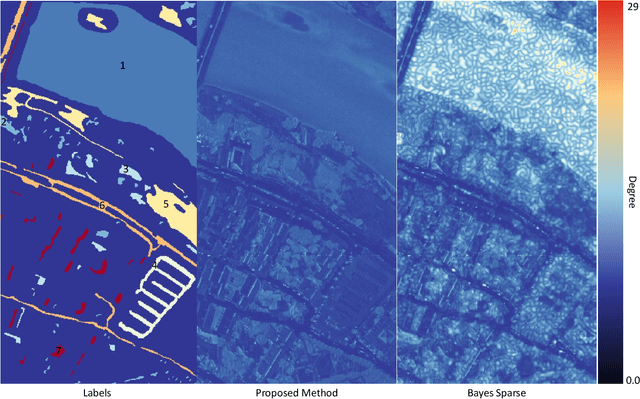
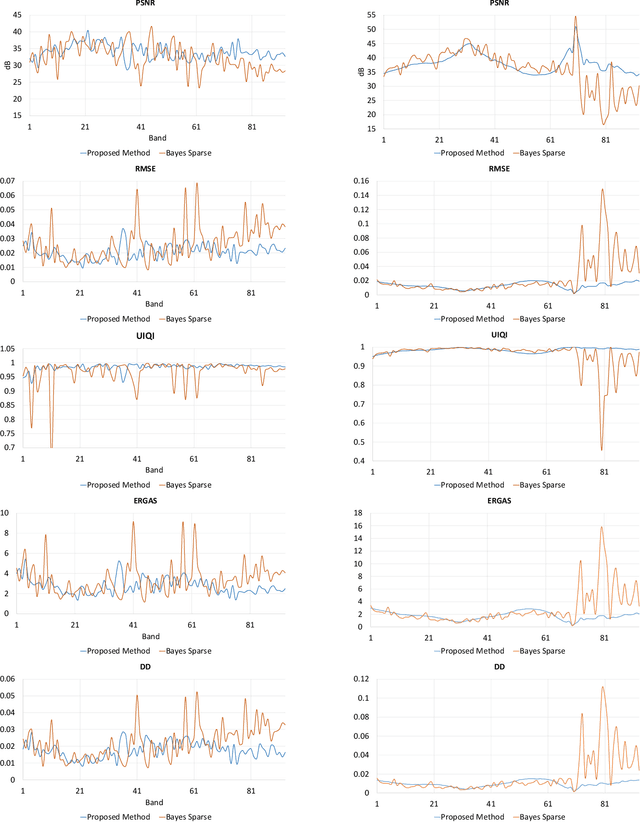

Abstract:Spectral imaging enables spatially-resolved identification of materials in remote sensing, biomedicine, and astronomy. However, acquisition times require balancing spectral and spatial resolution with signal-to-noise. Hyperspectral imaging provides superior material specificity, while multispectral images are faster to collect at greater fidelity. We propose an approach for fusing hyperspectral and multispectral images to provide high-quality hyperspectral output. The proposed optimization leverages the least absolute shrinkage and selection operator (LASSO) to perform variable selection and regularization. Computational time is reduced by applying the alternating direction method of multipliers (ADMM), as well as initializing the fusion image by estimating it using maximum a posteriori (MAP) based on Hardie's method. We demonstrate that the proposed sparse fusion and reconstruction provides quantitatively superior results when compared to existing methods on publicly available images. Finally, we show how the proposed method can be practically applied in biomedical infrared spectroscopic microscopy.
DVNet: A Memory-Efficient Three-Dimensional CNN for Large-Scale Neurovascular Reconstruction
Feb 04, 2020
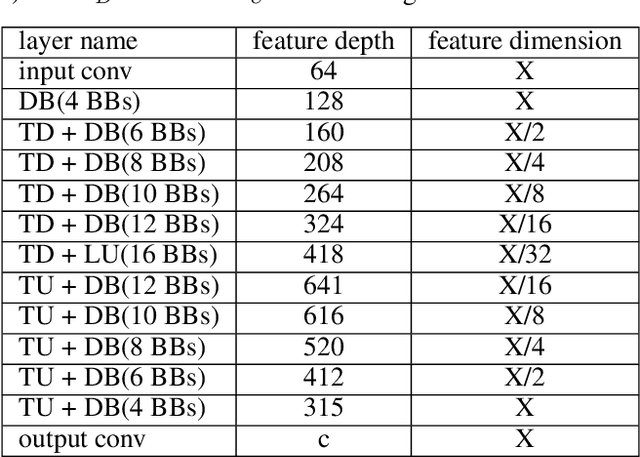


Abstract:Maps of brain microarchitecture are important for understanding neurological function and behavior, including alterations caused by chronic conditions such as neurodegenerative disease. Techniques such as knife-edge scanning microscopy (KESM) provide the potential for whole organ imaging at sub-cellular resolution. However, multi-terabyte data sizes make manual annotation impractical and automatic segmentation challenging. Densely packed cells combined with interconnected microvascular networks are a challenge for current segmentation algorithms. The massive size of high-throughput microscopy data necessitates fast and largely unsupervised algorithms. In this paper, we investigate a fully-convolutional, deep, and densely-connected encoder-decoder for pixel-wise semantic segmentation. The excessive memory complexity often encountered with deep and dense networks is mitigated using skip connections, resulting in fewer parameters and enabling a significant performance increase over prior architectures. The proposed network provides superior performance for semantic segmentation problems applied to open-source benchmarks. We finally demonstrate our network for cellular and microvascular segmentation, enabling quantitative metrics for organ-scale neurovascular analysis.
Three-Dimensional GPU-Accelerated Active Contours for Automated Localization of Cells in Large Images
Apr 17, 2018



Abstract:Cell segmentation in microscopy is a challenging problem, since cells are often asymmetric and densely packed. This becomes particularly challenging for extremely large images, since manual intervention and processing time can make segmentation intractable. In this paper, we present an efficient and highly parallel formulation for symmetric three-dimensional (3D) contour evolution that extends previous work on fast two-dimensional active contours. We provide a formulation for optimization on 3D images, as well as a strategy for accelerating computation on consumer graphics hardware. The proposed software takes advantage of Monte-Carlo sampling schemes in order to speed up convergence and reduce thread divergence. Experimental results show that this method provides superior performance for large 2D and 3D cell segmentation tasks when compared to existing methods on large 3D brain images.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge