David Coz
DermGAN: Synthetic Generation of Clinical Skin Images with Pathology
Nov 20, 2019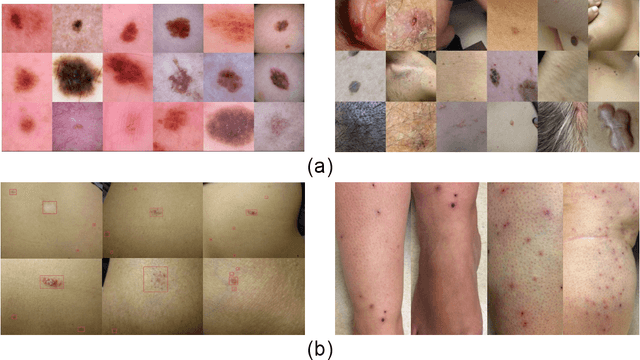

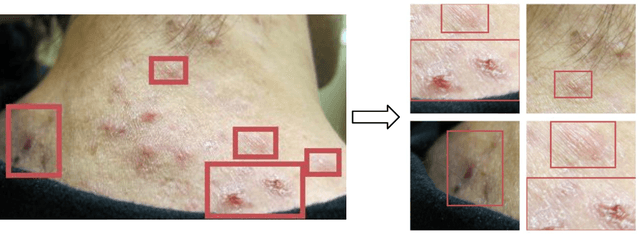
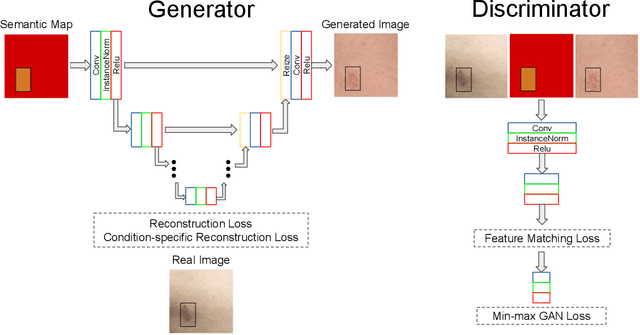
Abstract:Despite the recent success in applying supervised deep learning to medical imaging tasks, the problem of obtaining large and diverse expert-annotated datasets required for the development of high performant models remains particularly challenging. In this work, we explore the possibility of using Generative Adverserial Networks (GAN) to synthesize clinical images with skin condition. We propose DermGAN, an adaptation of the popular Pix2Pix architecture, to create synthetic images for a pre-specified skin condition while being able to vary its size, location and the underlying skin color. We demonstrate that the generated images are of high fidelity using objective GAN evaluation metrics. In a Human Turing test, we note that the synthetic images are not only visually similar to real images, but also embody the respective skin condition in dermatologists' eyes. Finally, when using the synthetic images as a data augmentation technique for training a skin condition classifier, we observe that the model performs comparably to the baseline model overall while improving on rare but malignant conditions.
A deep learning system for differential diagnosis of skin diseases
Sep 11, 2019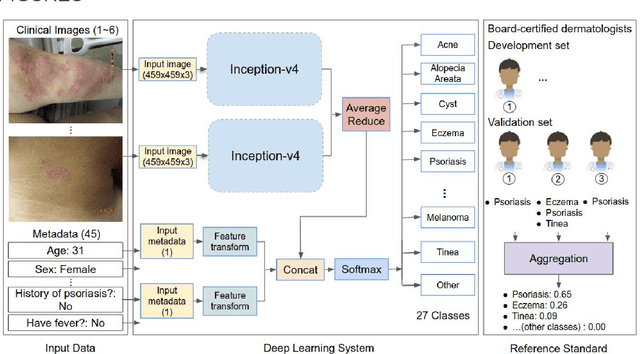
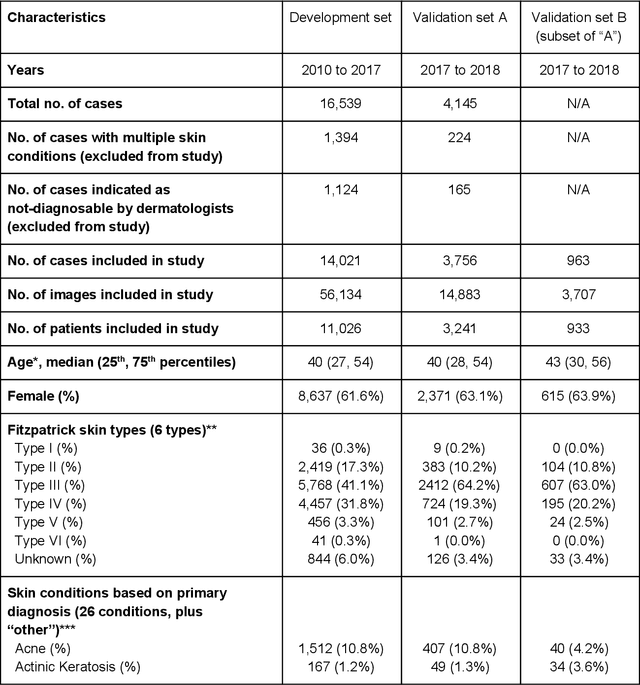
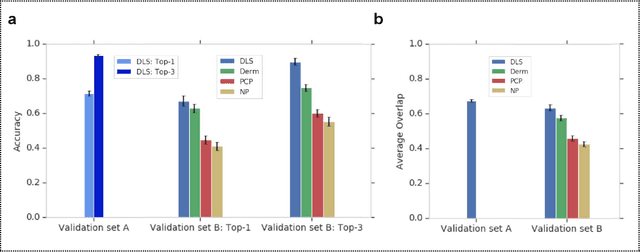
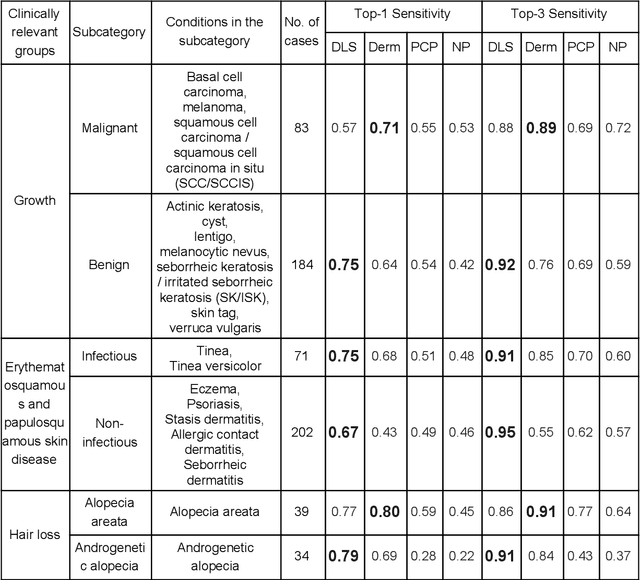
Abstract:Skin conditions affect an estimated 1.9 billion people worldwide. A shortage of dermatologists causes long wait times and leads patients to seek dermatologic care from general practitioners. However, the diagnostic accuracy of general practitioners has been reported to be only 0.24-0.70 (compared to 0.77-0.96 for dermatologists), resulting in referral errors, delays in care, and errors in diagnosis and treatment. In this paper, we developed a deep learning system (DLS) to provide a differential diagnosis of skin conditions for clinical cases (skin photographs and associated medical histories). The DLS distinguishes between 26 skin conditions that represent roughly 80% of the volume of skin conditions seen in primary care. The DLS was developed and validated using de-identified cases from a teledermatology practice serving 17 clinical sites via a temporal split: the first 14,021 cases for development and the last 3,756 cases for validation. On the validation set, where a panel of three board-certified dermatologists defined the reference standard for every case, the DLS achieved 0.71 and 0.93 top-1 and top-3 accuracies respectively. For a random subset of the validation set (n=963 cases), 18 clinicians reviewed the cases for comparison. On this subset, the DLS achieved a 0.67 top-1 accuracy, non-inferior to board-certified dermatologists (0.63, p<0.001), and higher than primary care physicians (PCPs, 0.45) and nurse practitioners (NPs, 0.41). The top-3 accuracy showed a similar trend: 0.90 DLS, 0.75 dermatologists, 0.60 PCPs, and 0.55 NPs. These results highlight the potential of the DLS to augment general practitioners to accurately diagnose skin conditions by suggesting differential diagnoses that may not have been considered. Future work will be needed to prospectively assess the clinical impact of using this tool in actual clinical workflows.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge