Chunlong Luo
DeepACC:Automate Chromosome Classification based on Metaphase Images using Deep Learning Framework Fused with Prior Knowledge
Jun 28, 2020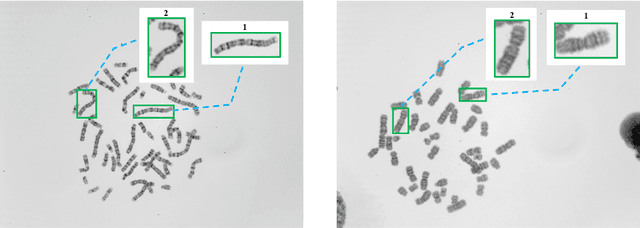
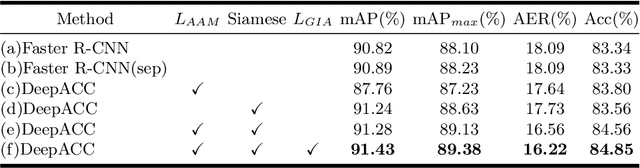
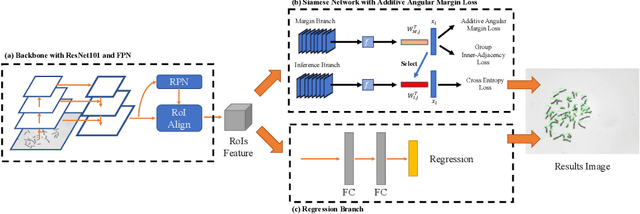

Abstract:Chromosome classification is an important but difficult and tedious task in karyotyping. Previous methods only classify manually segmented single chromosome, which is far from clinical practice. In this work, we propose a detection based method, DeepACC, to locate and fine classify chromosomes simultaneously based on the whole metaphase image. We firstly introduce the Additive Angular Margin Loss to enhance the discriminative power of model. To alleviate batch effects, we transform decision boundary of each class case-by-case through a siamese network which make full use of prior knowledges that chromosomes usually appear in pairs. Furthermore, we take the clinically seven group criterion as a prior knowledge and design an additional Group Inner-Adjacency Loss to further reduce inter-class similarities. 3390 metaphase images from clinical laboratory are collected and labelled to evaluate the performance. Results show that the new design brings encouraging performance gains comparing to the state-of-the-art baselines.
PBRnet: Pyramidal Bounding Box Refinement to Improve Object Localization Accuracy
Mar 10, 2020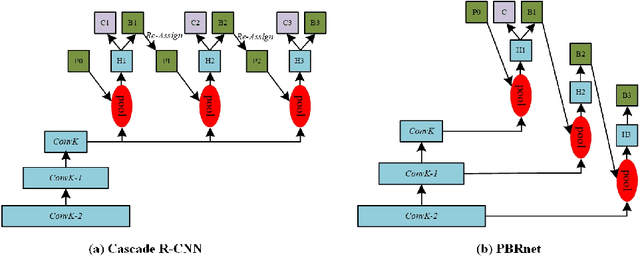
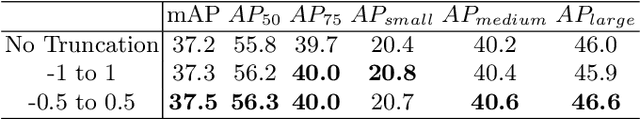
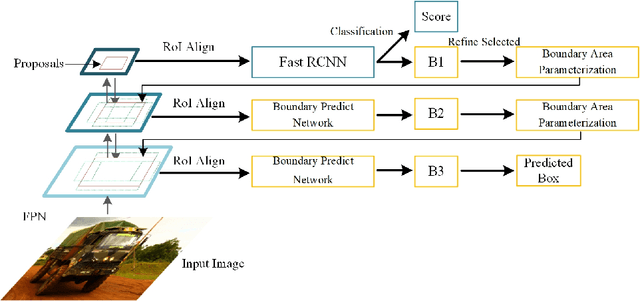
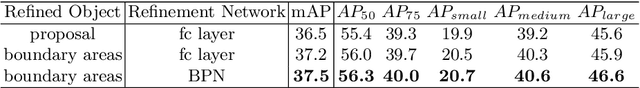
Abstract:Many recently developed object detectors focused on coarse-to-fine framework which contains several stages that classify and regress proposals from coarse-grain to fine-grain, and obtains more accurate detection gradually. Multi-resolution models such as Feature Pyramid Network(FPN) integrate information of different levels of resolution and effectively improve the performance. Previous researches also have revealed that localization can be further improved by: 1) using fine-grained information which is more translational variant; 2) refining local areas which is more focused on local boundary information. Based on these principles, we designed a novel boundary refinement architecture to improve localization accuracy by combining coarse-to-fine framework with feature pyramid structure, named as Pyramidal Bounding Box Refinement network(PBRnet), which parameterizes gradually focused boundary areas of objects and leverages lower-level feature maps to extract finer local information when refining the predicted bounding boxes. Extensive experiments are performed on the MS-COCO dataset. The PBRnet brings a significant performance gains by roughly 3 point of $mAP$ when added to FPN or Libra R-CNN. Moreover, by treating Cascade R-CNN as a coarse-to-fine detector and replacing its localization branch by the regressor of PBRnet, it leads an extra performance improvement by 1.5 $mAP$, yielding a total performance boosting by as high as 5 point of $mAP$.
Learning from Suspected Target: Bootstrapping Performance for Breast Cancer Detection in Mammography
Mar 01, 2020



Abstract:Deep learning object detection algorithm has been widely used in medical image analysis. Currently all the object detection tasks are based on the data annotated with object classes and their bounding boxes. On the other hand, medical images such as mammography usually contain normal regions or objects that are similar to the lesion region, and may be misclassified in the testing stage if they are not taken care of. In this paper, we address such problem by introducing a novel top likelihood loss together with a new sampling procedure to select and train the suspected target regions, as well as proposing a similarity loss to further identify suspected targets from targets. Mean average precision (mAP) according to the predicted targets and specificity, sensitivity, accuracy, AUC values according to classification of patients are adopted for performance comparisons. We firstly test our proposed method on a private dense mammogram dataset. Results show that our proposed method greatly reduce the false positive rate and the specificity is increased by 0.25 on detecting mass type cancer. It is worth mention that dense breast typically has a higher risk for developing breast cancers and also are harder for cancer detection in diagnosis, and our method outperforms a reported result from performance of radiologists. Our method is also validated on the public Digital Database for Screening Mammography (DDSM) dataset, brings significant improvement on mass type cancer detection and outperforms the most state-of-the-art work.
DeepACE: Automated Chromosome Enumeration in Metaphase Cell Images Using Deep Convolutional Neural Networks
Oct 12, 2019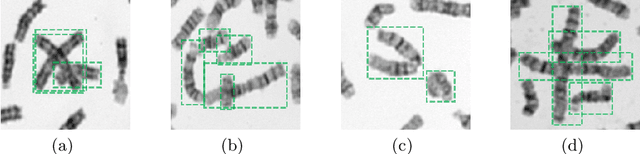
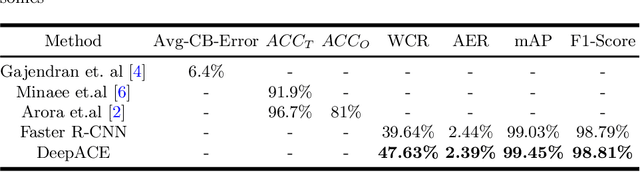
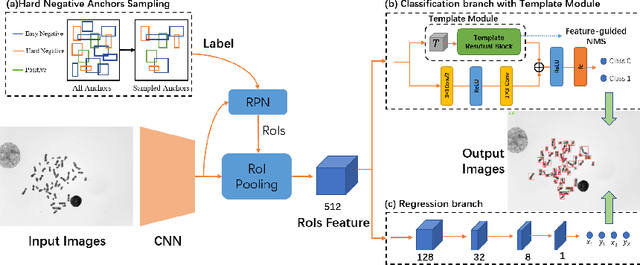
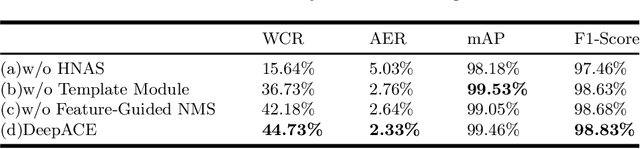
Abstract:Chromosome enumeration is an important but tedious procedure in karyotyping analysis. In this paper, to automate the enumeration process, we developed a chromosome enumeration framework, DeepACE, based on the region based object detection scheme. Firstly, the ability of region proposal network is enhanced by a newly proposed Hard Negative Anchors Sampling to extract unapparent but important information about highly confusing partial chromosomes. Next, to alleviate serious occlusion problems, we novelly introduced a weakly-supervised mechanism by adding a Template Module into classification branch to heuristically separate overlapped chromosomes. The template features are further incorporated into the NMS procedure to further improve the detection of overlapping chromosomes. In the newly collected clinical dataset, the proposed method outperform all the previous method, yielding an mAP with respect to chromosomes as 99.45, and the error rate is about 2.4%.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge