Christian Langkammer
Identifying Alzheimer's Disease Prediction Strategies of Convolutional Neural Network Classifiers using R2* Maps and Spectral Clustering
Jun 04, 2025Abstract:Deep learning models have shown strong performance in classifying Alzheimer's disease (AD) from R2* maps, but their decision-making remains opaque, raising concerns about interpretability. Previous studies suggest biases in model decisions, necessitating further analysis. This study uses Layer-wise Relevance Propagation (LRP) and spectral clustering to explore classifier decision strategies across preprocessing and training configurations using R2* maps. We trained a 3D convolutional neural network on R2* maps, generating relevance heatmaps via LRP and applied spectral clustering to identify dominant patterns. t-Stochastic Neighbor Embedding (t-SNE) visualization was used to assess clustering structure. Spectral clustering revealed distinct decision patterns, with the relevance-guided model showing the clearest separation between AD and normal control (NC) cases. The t-SNE visualization confirmed that this model aligned heatmap groupings with the underlying subject groups. Our findings highlight the significant impact of preprocessing and training choices on deep learning models trained on R2* maps, even with similar performance metrics. Spectral clustering offers a structured method to identify classification strategy differences, emphasizing the importance of explainability in medical AI.
Pfungst and Clever Hans: Identifying the unintended cues in a widely used Alzheimer's disease MRI dataset using explainable deep learning
Jan 27, 2025



Abstract:Backgrounds. Deep neural networks have demonstrated high accuracy in classifying Alzheimer's disease (AD). This study aims to enlighten the underlying black-box nature and reveal individual contributions of T1-weighted (T1w) gray-white matter texture, volumetric information and preprocessing on classification performance. Methods. We utilized T1w MRI data from the Alzheimer's Disease Neuroimaging Initiative to distinguish matched AD patients (990 MRIs) from healthy controls (990 MRIs). Preprocessing included skull stripping and binarization at varying thresholds to systematically eliminate texture information. A deep neural network was trained on these configurations, and the model performance was compared using McNemar tests with discrete Bonferroni-Holm correction. Layer-wise Relevance Propagation (LRP) and structural similarity metrics between heatmaps were applied to analyze learned features. Results. Classification performance metrics (accuracy, sensitivity, and specificity) were comparable across all configurations, indicating a negligible influence of T1w gray- and white signal texture. Models trained on binarized images demonstrated similar feature performance and relevance distributions, with volumetric features such as atrophy and skull-stripping features emerging as primary contributors. Conclusions. We revealed a previously undiscovered Clever Hans effect in a widely used AD MRI dataset. Deep neural networks classification predominantly rely on volumetric features, while eliminating gray-white matter T1w texture did not decrease the performance. This study clearly demonstrates an overestimation of the importance of gray-white matter contrasts, at least for widely used structural T1w images, and highlights potential misinterpretation of performance metrics.
Explainable concept mappings of MRI: Revealing the mechanisms underlying deep learning-based brain disease classification
Apr 16, 2024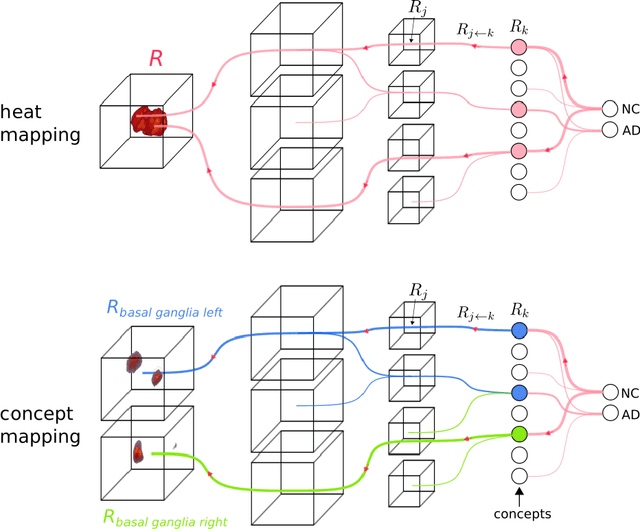
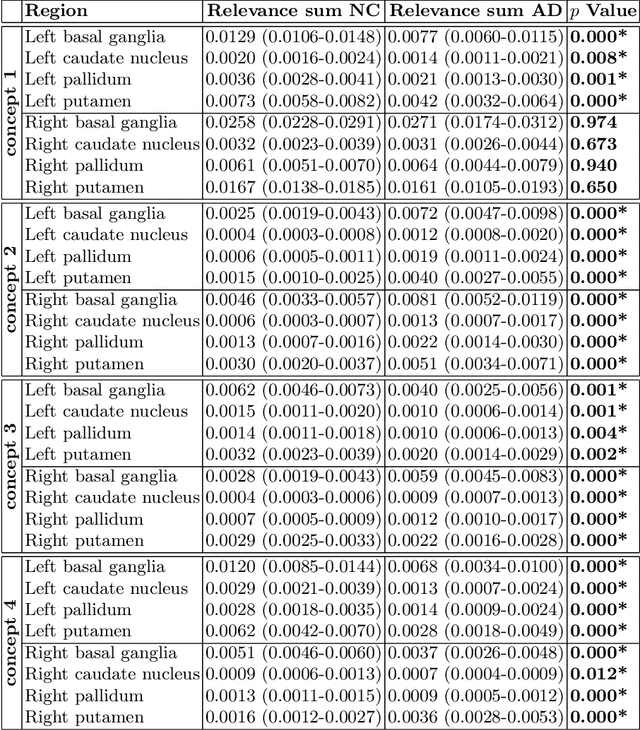
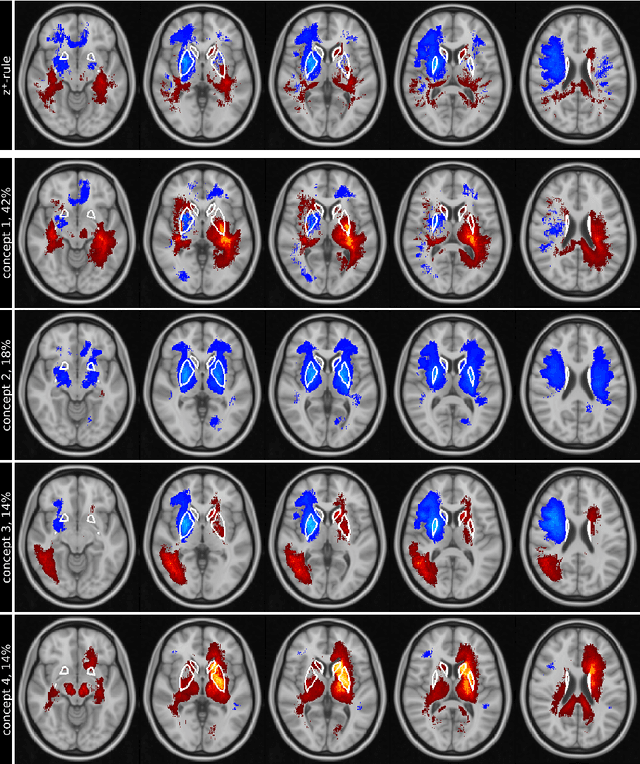
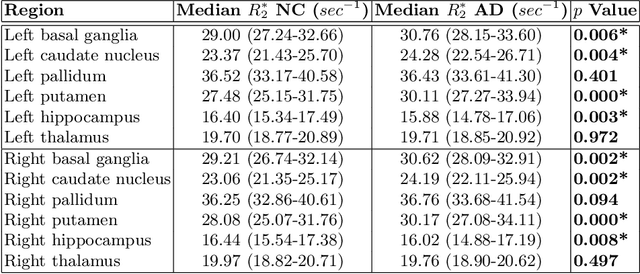
Abstract:Motivation. While recent studies show high accuracy in the classification of Alzheimer's disease using deep neural networks, the underlying learned concepts have not been investigated. Goals. To systematically identify changes in brain regions through concepts learned by the deep neural network for model validation. Approach. Using quantitative R2* maps we separated Alzheimer's patients (n=117) from normal controls (n=219) by using a convolutional neural network and systematically investigated the learned concepts using Concept Relevance Propagation and compared these results to a conventional region of interest-based analysis. Results. In line with established histological findings and the region of interest-based analyses, highly relevant concepts were primarily found in and adjacent to the basal ganglia. Impact. The identification of concepts learned by deep neural networks for disease classification enables validation of the models and could potentially improve reliability.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge