Changcai Yang
CausalCellSegmenter: Causal Inference inspired Diversified Aggregation Convolution for Pathology Image Segmentation
Mar 10, 2024Abstract:Deep learning models have shown promising performance for cell nucleus segmentation in the field of pathology image analysis. However, training a robust model from multiple domains remains a great challenge for cell nucleus segmentation. Additionally, the shortcomings of background noise, highly overlapping between cell nucleus, and blurred edges often lead to poor performance. To address these challenges, we propose a novel framework termed CausalCellSegmenter, which combines Causal Inference Module (CIM) with Diversified Aggregation Convolution (DAC) techniques. The DAC module is designed which incorporates diverse downsampling features through a simple, parameter-free attention module (SimAM), aiming to overcome the problems of false-positive identification and edge blurring. Furthermore, we introduce CIM to leverage sample weighting by directly removing the spurious correlations between features for every input sample and concentrating more on the correlation between features and labels. Extensive experiments on the MoNuSeg-2018 dataset achieves promising results, outperforming other state-of-the-art methods, where the mIoU and DSC scores growing by 3.6% and 2.65%.
OpenNDD: Open Set Recognition for Neurodevelopmental Disorders Detection
Jun 28, 2023Abstract:Neurodevelopmental disorders (NDDs) are a highly prevalent group of disorders and represent strong clinical behavioral similarities, and that make it very challenging for accurate identification of different NDDs such as autism spectrum disorder (ASD) and attention-deficit hyperactivity disorder (ADHD). Moreover, there is no reliable physiological markers for NDDs diagnosis and it solely relies on psychological evaluation criteria. However, it is crucial to prevent misdiagnosis and underdiagnosis by intelligent assisted diagnosis, which is closely related to the follow-up corresponding treatment. In order to relieve these issues, we propose a novel open set recognition framework for NDDs screening and detection, which is the first application of open set recognition in this field. It combines auto encoder and adversarial reciprocal points open set recognition to accurately identify known classes as well as recognize classes never encountered. And considering the strong similarities between different subjects, we present a joint scaling method called MMS to distinguish unknown disorders. To validate the feasibility of our presented method, we design a reciprocal opposition experiment protocol on the hybrid datasets from Autism Brain Imaging Data Exchange I (ABIDE I) and THE ADHD-200 SAMPLE (ADHD-200) with 791 samples from four sites and the results demonstrate the superiority on various metrics. Our OpenNDD has achieved promising performance, where the accuracy is 77.38%, AUROC is 75.53% and the open set classification rate is as high as 59.43%.
MC-UNet Multi-module Concatenation based on U-shape Network for Retinal Blood Vessels Segmentation
Apr 07, 2022
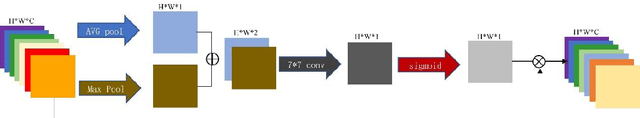
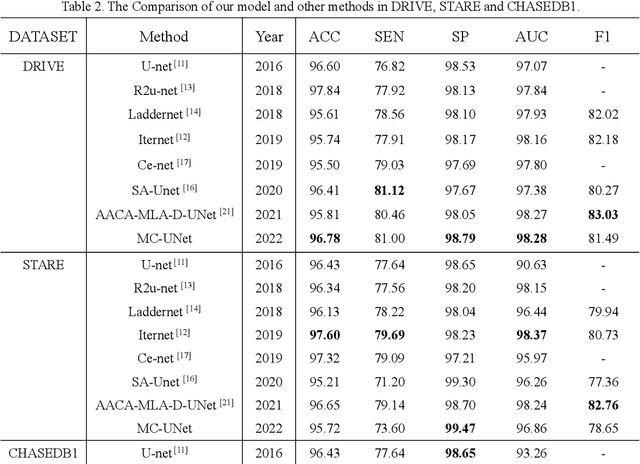
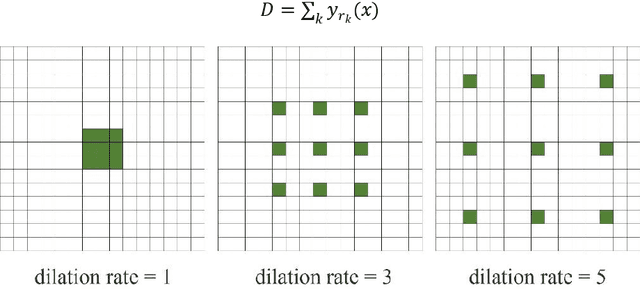
Abstract:Accurate segmentation of the blood vessels of the retina is an important step in clinical diagnosis of ophthalmic diseases. Many deep learning frameworks have come up for retinal blood vessels segmentation tasks. However, the complex vascular structure and uncertain pathological features make the blood vessel segmentation still very challenging. A novel U-shaped network named Multi-module Concatenation which is based on Atrous convolution and multi-kernel pooling is put forward to retinal vessels segmentation in this paper. The proposed network structure retains three layers the essential structure of U-Net, in which the atrous convolution combining the multi-kernel pooling blocks are designed to obtain more contextual information. The spatial attention module is concatenated with dense atrous convolution module and multi-kernel pooling module to form a multi-module concatenation. And different dilation rates are selected by cascading to acquire a larger receptive field in atrous convolution. Adequate comparative experiments are conducted on these public retinal datasets: DRIVE, STARE and CHASE_DB1. The results show that the proposed method is effective, especially for microvessels. The code will be put out at https://github.com/Rebeccala/MC-UNet
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge