Brent Mombourquette
Whiterabbit AI, Inc., 3930 Freedom Circle Suite 101, Santa Clara, CA 95054
Problems and shortcuts in deep learning for screening mammography
Mar 29, 2023Abstract:This work reveals undiscovered challenges in the performance and generalizability of deep learning models. We (1) identify spurious shortcuts and evaluation issues that can inflate performance and (2) propose training and analysis methods to address them. We trained an AI model to classify cancer on a retrospective dataset of 120,112 US exams (3,467 cancers) acquired from 2008 to 2017 and 16,693 UK exams (5,655 cancers) acquired from 2011 to 2015. We evaluated on a screening mammography test set of 11,593 US exams (102 cancers; 7,594 women; age 57.1 \pm 11.0) and 1,880 UK exams (590 cancers; 1,745 women; age 63.3 \pm 7.2). A model trained on images of only view markers (no breast) achieved a 0.691 AUC. The original model trained on both datasets achieved a 0.945 AUC on the combined US+UK dataset but paradoxically only 0.838 and 0.892 on the US and UK datasets, respectively. Sampling cancers equally from both datasets during training mitigated this shortcut. A similar AUC paradox (0.903) occurred when evaluating diagnostic exams vs screening exams (0.862 vs 0.861, respectively). Removing diagnostic exams during training alleviated this bias. Finally, the model did not exhibit the AUC paradox over scanner models but still exhibited a bias toward Selenia Dimension (SD) over Hologic Selenia (HS) exams. Analysis showed that this AUC paradox occurred when a dataset attribute had values with a higher cancer prevalence (dataset bias) and the model consequently assigned a higher probability to these attribute values (model bias). Stratification and balancing cancer prevalence can mitigate shortcuts during evaluation. Dataset and model bias can introduce shortcuts and the AUC paradox, potentially pervasive issues within the healthcare AI space. Our methods can verify and mitigate shortcuts while providing a clear understanding of performance.
Deep is a Luxury We Don't Have
Aug 11, 2022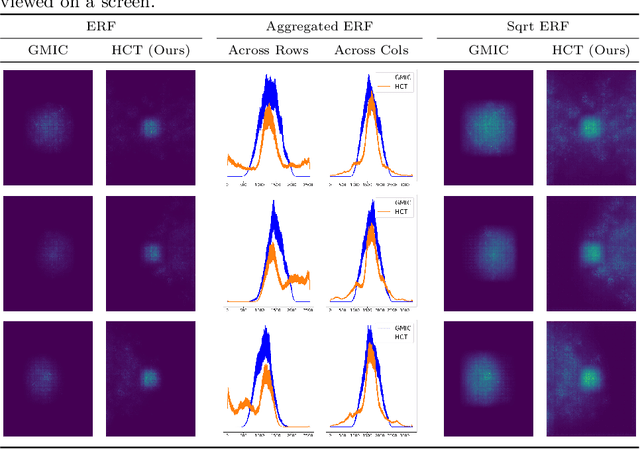
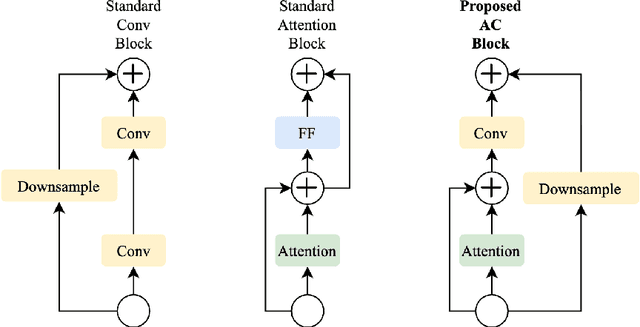
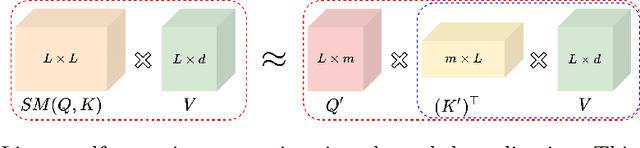

Abstract:Medical images come in high resolutions. A high resolution is vital for finding malignant tissues at an early stage. Yet, this resolution presents a challenge in terms of modeling long range dependencies. Shallow transformers eliminate this problem, but they suffer from quadratic complexity. In this paper, we tackle this complexity by leveraging a linear self-attention approximation. Through this approximation, we propose an efficient vision model called HCT that stands for High resolution Convolutional Transformer. HCT brings transformers' merits to high resolution images at a significantly lower cost. We evaluate HCT using a high resolution mammography dataset. HCT is significantly superior to its CNN counterpart. Furthermore, we demonstrate HCT's fitness for medical images by evaluating its effective receptive field.Code available at https://bit.ly/3ykBhhf
A deep learning algorithm for reducing false positives in screening mammography
Apr 13, 2022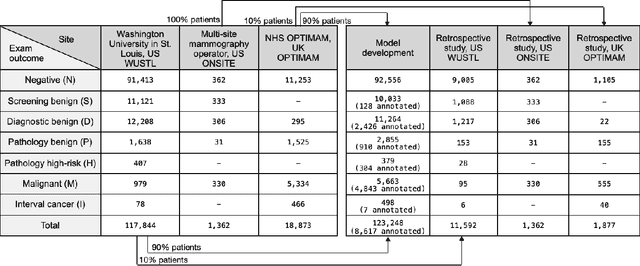
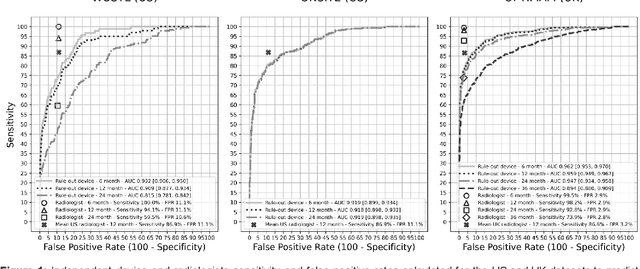
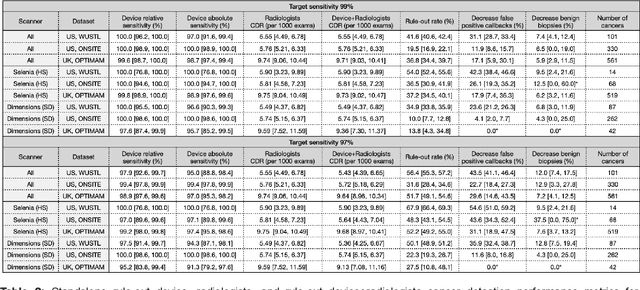
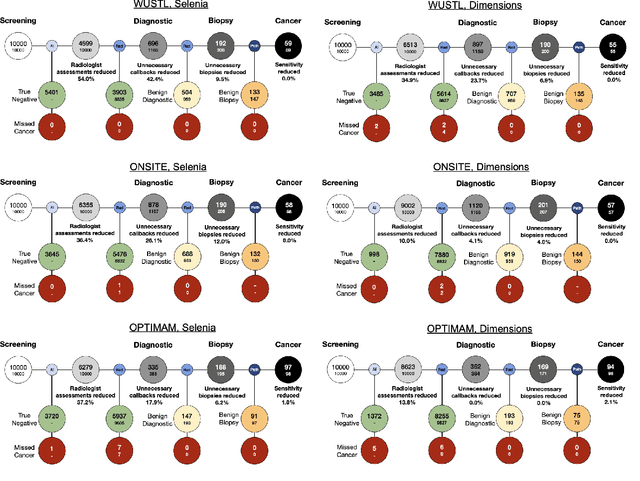
Abstract:Screening mammography improves breast cancer outcomes by enabling early detection and treatment. However, false positive callbacks for additional imaging from screening exams cause unnecessary procedures, patient anxiety, and financial burden. This work demonstrates an AI algorithm that reduces false positives by identifying mammograms not suspicious for breast cancer. We trained the algorithm to determine the absence of cancer using 123,248 2D digital mammograms (6,161 cancers) and performed a retrospective study on 14,831 screening exams (1,026 cancers) from 15 US and 3 UK sites. Retrospective evaluation of the algorithm on the largest of the US sites (11,592 mammograms, 101 cancers) a) left the cancer detection rate unaffected (p=0.02, non-inferiority margin 0.25 cancers per 1000 exams), b) reduced callbacks for diagnostic exams by 31.1% compared to standard clinical readings, c) reduced benign needle biopsies by 7.4%, and d) reduced screening exams requiring radiologist interpretation by 41.6% in the simulated clinical workflow. This work lays the foundation for semi-autonomous breast cancer screening systems that could benefit patients and healthcare systems by reducing false positives, unnecessary procedures, patient anxiety, and expenses.
A multi-site study of a breast density deep learning model for full-field digital mammography and digital breast tomosynthesis exams
Jan 23, 2020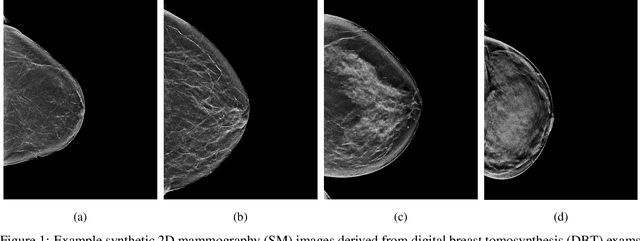
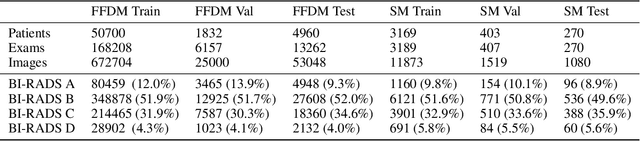
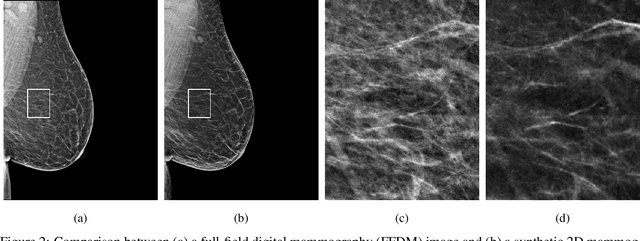
Abstract:$\textbf{Purpose:}$ To develop a Breast Imaging Reporting and Data System (BI-RADS) breast density DL model in a multi-site setting for synthetic 2D mammography (SM) images derived from 3D DBT exams using FFDM images and limited SM data. $\textbf{Materials and Methods:}$ A DL model was trained to predict BI-RADS breast density using FFDM images acquired from 2008 to 2017 (Site 1: 57492 patients, 187627 exams, 750752 images) for this retrospective study. The FFDM model was evaluated using SM datasets from two institutions (Site 1: 3842 patients, 3866 exams, 14472 images, acquired from 2016 to 2017; Site 2: 7557 patients, 16283 exams, 63973 images, 2015 to 2019). Adaptation methods were investigated to improve performance on the SM datasets and the effect of dataset size on each adaptation method is considered. Statistical significance was assessed using confidence intervals (CI), estimated by bootstrapping. $\textbf{Results:}$ Without adaptation, the model demonstrated close agreement with the original reporting radiologists for all three datasets (Site 1 FFDM: linearly-weighted $\kappa_w$ = 0.75, 95\% CI: [0.74, 0.76]; Site 1 SM: $\kappa_w$ = 0.71, CI: [0.64, 0.78]; Site 2 SM: $\kappa_w$ = 0.72, CI: [0.70, 0.75]). With adaptation, performance improved for Site 2 (Site 1: $\kappa_w$ = 0.72, CI: [0.66, 0.79], Site 2: $\kappa_w$ = 0.79, CI: [0.76, 0.81]) using only 500 SM images from each site. $\textbf{Conclusion:}$ A BI-RADS breast density DL model demonstrated strong performance on FFDM and SM images from two institutions without training on SM images and improved using few SM images.
A Hypersensitive Breast Cancer Detector
Jan 23, 2020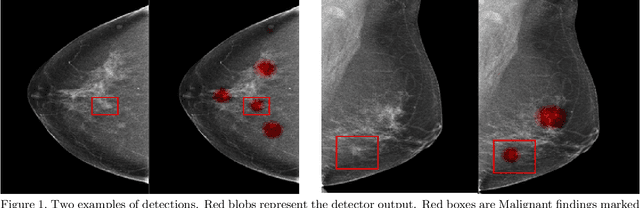
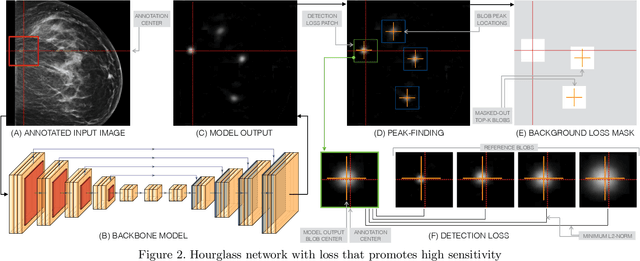
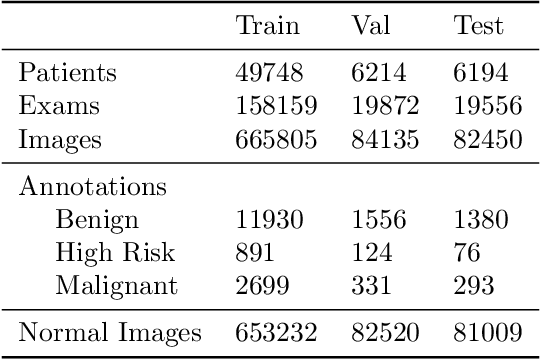
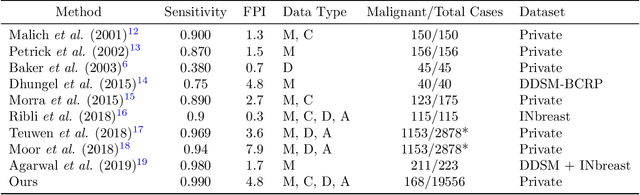
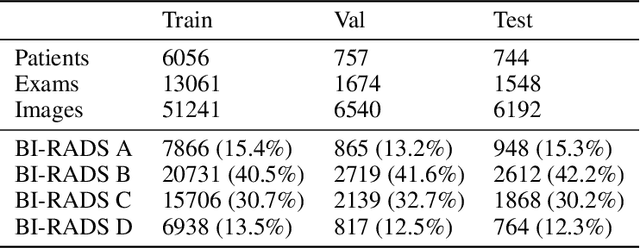
Abstract:Early detection of breast cancer through screening mammography yields a 20-35% increase in survival rate; however, there are not enough radiologists to serve the growing population of women seeking screening mammography. Although commercial computer aided detection (CADe) software has been available to radiologists for decades, it has failed to improve the interpretation of full-field digital mammography (FFDM) images due to its low sensitivity over the spectrum of findings. In this work, we leverage a large set of FFDM images with loose bounding boxes of mammographically significant findings to train a deep learning detector with extreme sensitivity. Building upon work from the Hourglass architecture, we train a model that produces segmentation-like images with high spatial resolution, with the aim of producing 2D Gaussian blobs centered on ground-truth boxes. We replace the pixel-wise $L_2$ norm with a weak-supervision loss designed to achieve high sensitivity, asymmetrically penalizing false positives and false negatives while softening the noise of the loose bounding boxes by permitting a tolerance in misaligned predictions. The resulting system achieves a sensitivity for malignant findings of 0.99 with only 4.8 false positive markers per image. When utilized in a CADe system, this model could enable a novel workflow where radiologists can focus their attention with trust on only the locations proposed by the model, expediting the interpretation process and bringing attention to potential findings that could otherwise have been missed. Due to its nearly perfect sensitivity, the proposed detector can also be used as a high-performance proposal generator in two-stage detection systems.
Adaptation of a deep learning malignancy model from full-field digital mammography to digital breast tomosynthesis
Jan 23, 2020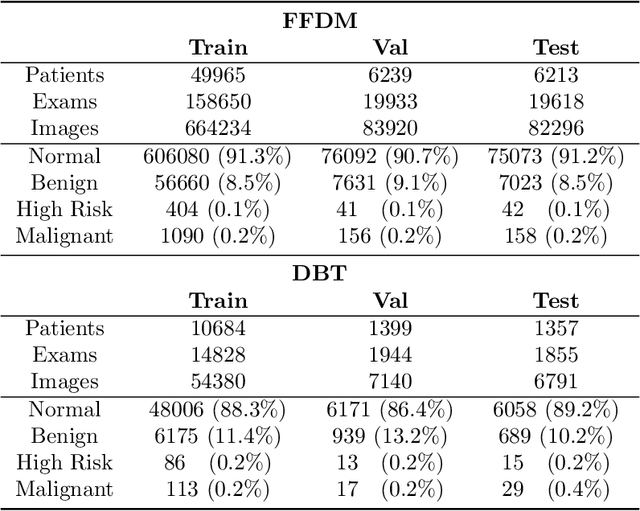
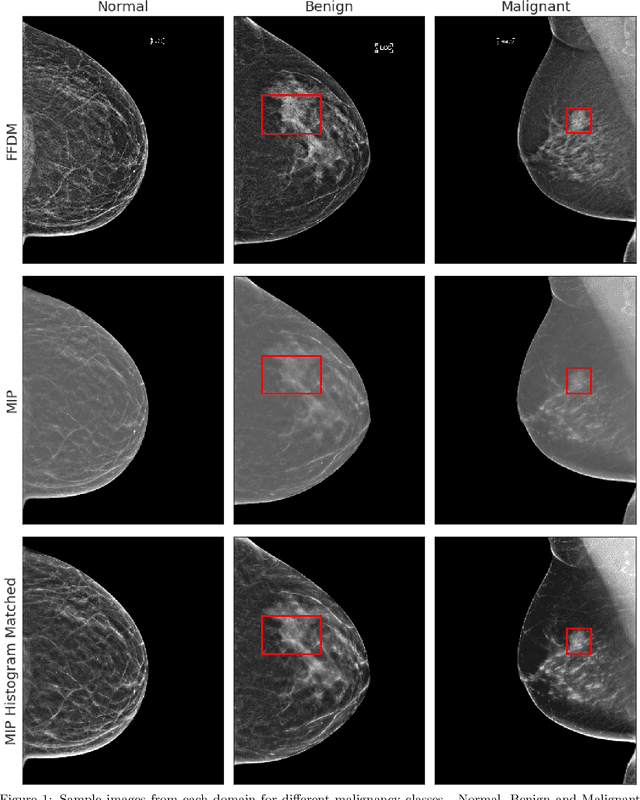
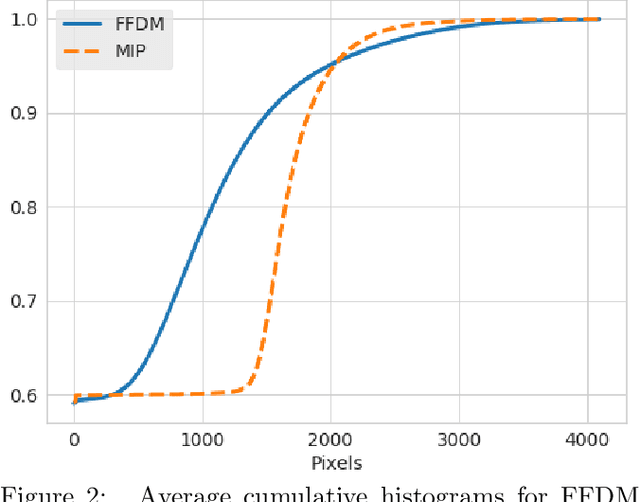
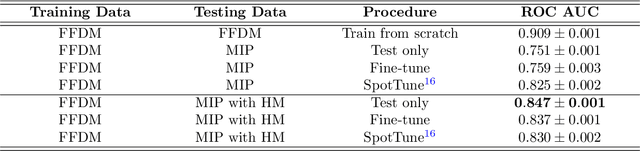
Abstract:Mammography-based screening has helped reduce the breast cancer mortality rate, but has also been associated with potential harms due to low specificity, leading to unnecessary exams or procedures, and low sensitivity. Digital breast tomosynthesis (DBT) improves on conventional mammography by increasing both sensitivity and specificity and is becoming common in clinical settings. However, deep learning (DL) models have been developed mainly on conventional 2D full-field digital mammography (FFDM) or scanned film images. Due to a lack of large annotated DBT datasets, it is difficult to train a model on DBT from scratch. In this work, we present methods to generalize a model trained on FFDM images to DBT images. In particular, we use average histogram matching (HM) and DL fine-tuning methods to generalize a FFDM model to the 2D maximum intensity projection (MIP) of DBT images. In the proposed approach, the differences between the FFDM and DBT domains are reduced via HM and then the base model, which was trained on abundant FFDM images, is fine-tuned. When evaluating on image patches extracted around identified findings, we are able to achieve similar areas under the receiver operating characteristic curve (ROC AUC) of $\sim 0.9$ for FFDM and $\sim 0.85$ for MIP images, as compared to a ROC AUC of $\sim 0.75$ when tested directly on MIP images.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge