Bhavna Antony
Uncertainty guided semi-supervised segmentation of retinal layers in OCT images
Mar 02, 2021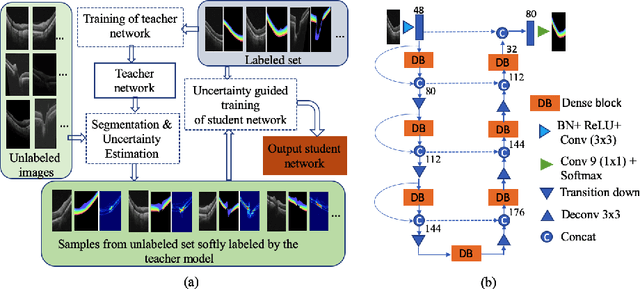
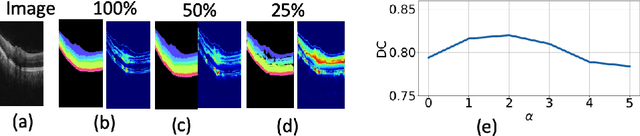
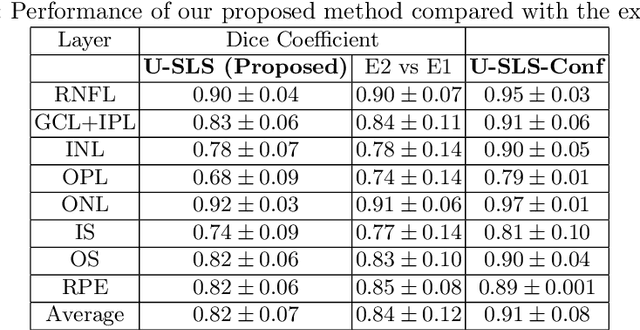
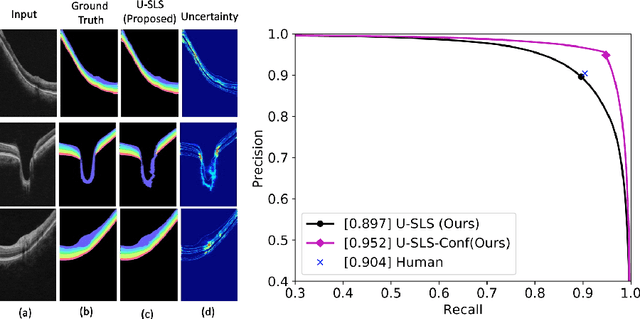
Abstract:Deep convolutional neural networks have shown outstanding performance in medical image segmentation tasks. The usual problem when training supervised deep learning methods is the lack of labeled data which is time-consuming and costly to obtain. In this paper, we propose a novel uncertainty-guided semi-supervised learning based on a student-teacher approach for training the segmentation network using limited labeled samples and a large number of unlabeled images. First, a teacher segmentation model is trained from the labeled samples using Bayesian deep learning. The trained model is used to generate soft segmentation labels and uncertainty maps for the unlabeled set. The student model is then updated using the softly segmented samples and the corresponding pixel-wise confidence of the segmentation quality estimated from the uncertainty of the teacher model using a newly designed loss function. Experimental results on a retinal layer segmentation task show that the proposed method improves the segmentation performance in comparison to the fully supervised approach and is on par with the expert annotator. The proposed semi-supervised segmentation framework is a key contribution and applicable for biomedical image segmentation across various imaging modalities where access to annotated medical images is challenging
* MICCAI,19
Inference of visual field test performance from OCT volumes using deep learning
Aug 26, 2019

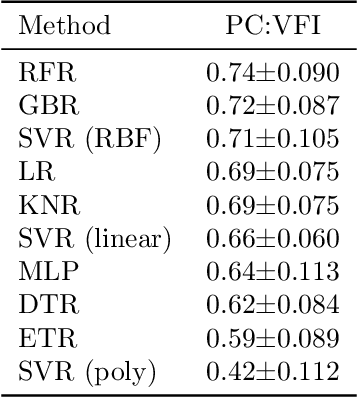

Abstract:Visual field tests (VFT) are pivotal for glaucoma diagnosis and conducted regularly to monitor disease progression. Here we address the question to what degree aggregate VFT measurements such as Visual Field Index (VFI) and Mean Deviation (MD) can be inferred from Optical Coherence Tomography (OCT) scans of the Optic Nerve Head (ONH) or the macula. Accurate inference of VFT measurements from OCT could reduce examination time and cost. We propose a novel 3D Convolutional Neural Network (CNN) for this task and compare its accuracy with classical machine learning (ML) algorithms trained on common, segmentation-based OCT, features employed for glaucoma diagnostics. Peak accuracies were achieved on ONH scans when inferring VFI with a Pearson Correlation (PC) of 0.88$\pm$0.035 for the CNN and a significantly lower (p $<$ 0.01) PC of 0.74$\pm$0.090 for the best performing, classical ML algorithm - a Random Forest regressor. Estimation of MD was equally accurate with a PC of 0.88$\pm$0.023 on ONH scans for the CNN.
Joint Segmentation and Uncertainty Visualization of Retinal Layers in Optical Coherence Tomography Images using Bayesian Deep Learning
Sep 12, 2018


Abstract:Optical coherence tomography (OCT) is commonly used to analyze retinal layers for assessment of ocular diseases. In this paper, we propose a method for retinal layer segmentation and quantification of uncertainty based on Bayesian deep learning. Our method not only performs end-to-end segmentation of retinal layers, but also gives the pixel wise uncertainty measure of the segmentation output. The generated uncertainty map can be used to identify erroneously segmented image regions which is useful in downstream analysis. We have validated our method on a dataset of 1487 images obtained from 15 subjects (OCT volumes) and compared it against the state-of-the-art segmentation algorithms that does not take uncertainty into account. The proposed uncertainty based segmentation method results in comparable or improved performance, and most importantly is more robust against noise.
A feature agnostic approach for glaucoma detection in OCT volumes
Aug 15, 2018
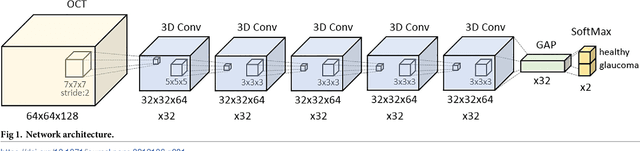

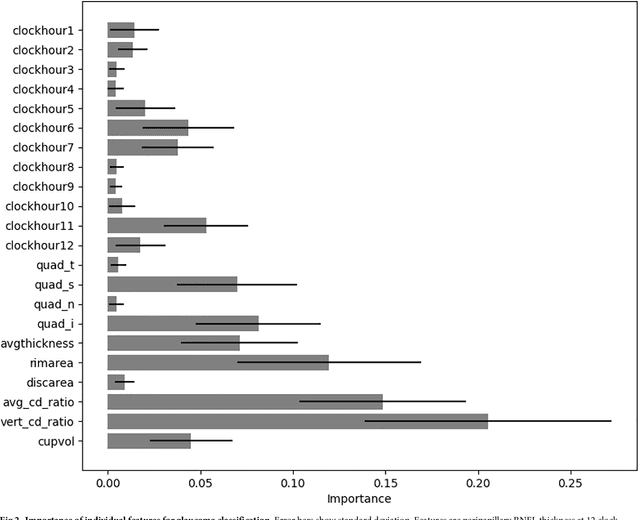
Abstract:Optical coherence tomography (OCT) based measurements of retinal layer thickness, such as the retinal nerve fibre layer (RNFL) and the ganglion cell with inner plexiform layer (GCIPL) are commonly used for the diagnosis and monitoring of glaucoma. Previously, machine learning techniques have utilized segmentation-based imaging features such as the peripapillary RNFL thickness and the cup-to-disc ratio. Here, we propose a deep learning technique that classifies eyes as healthy or glaucomatous directly from raw, unsegmented OCT volumes of the optic nerve head (ONH) using a 3D Convolutional Neural Network (CNN). We compared the accuracy of this technique with various feature-based machine learning algorithms and demonstrated the superiority of the proposed deep learning based method. Logistic regression was found to be the best performing classical machine learning technique with an AUC of 0.89. In direct comparison, the deep learning approach achieved a substantially higher AUC of 0.94 with the additional advantage of providing insight into which regions of an OCT volume are important for glaucoma detection. Computing Class Activation Maps (CAM), we found that the CNN identified neuroretinal rim and optic disc cupping as well as the lamina cribrosa (LC) and its surrounding areas as the regions significantly associated with the glaucoma classification. These regions anatomically correspond to the well established and commonly used clinical markers for glaucoma diagnosis such as increased cup volume, cup diameter, and neuroretinal rim thinning at the superior and inferior segments.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge