Bas Veeling
Supervised Uncertainty Quantification for Segmentation with Multiple Annotations
Jul 03, 2019


Abstract:The accurate estimation of predictive uncertainty carries importance in medical scenarios such as lung node segmentation. Unfortunately, most existing works on predictive uncertainty do not return calibrated uncertainty estimates, which could be used in practice. In this work we exploit multi-grader annotation variability as a source of 'groundtruth' aleatoric uncertainty, which can be treated as a target in a supervised learning problem. We combine this groundtruth uncertainty with a Probabilistic U-Net and test on the LIDC-IDRI lung nodule CT dataset and MICCAI2012 prostate MRI dataset. We find that we are able to improve predictive uncertainty estimates. We also find that we can improve sample accuracy and sample diversity.
Towards radiologist-level cancer risk assessment in CT lung screening using deep learning
Apr 05, 2018
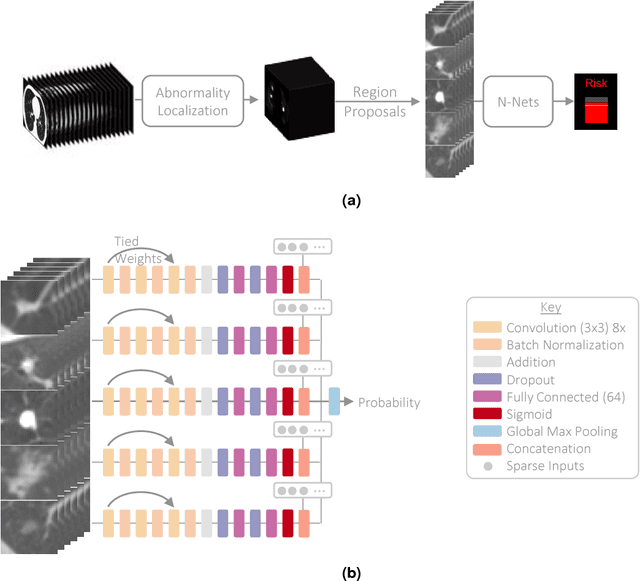
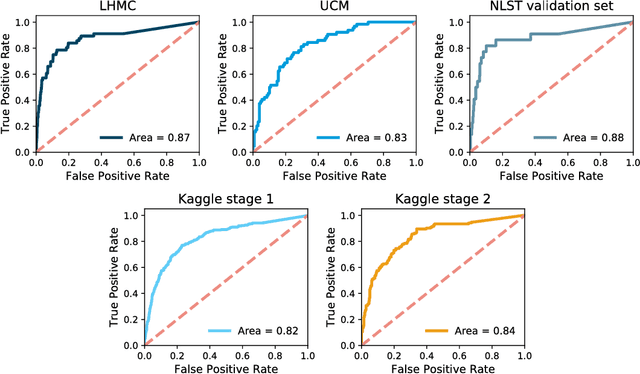
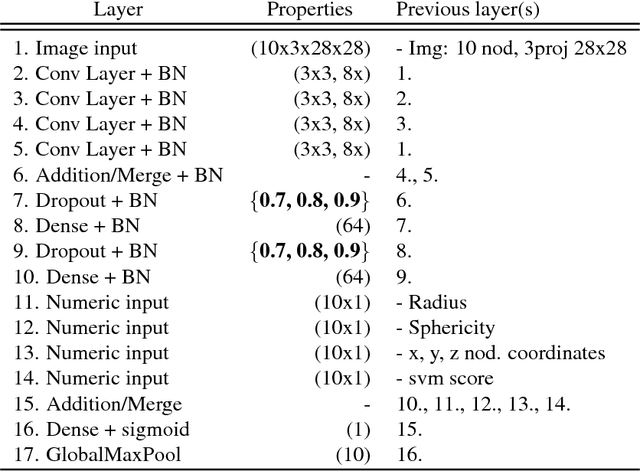
Abstract:Lung cancer is the leading cause of cancer mortality in the US, responsible for more deaths than breast, prostate, colon and pancreas cancer combined. Recently, it has been demonstrated that screening those at high-risk for lung cancer low-dose computed tomography (CT) of the chest can significantly reduce this death rate. The process of evaluating a chest CT scan involves the identification of nodules that are contained within a scan as well as the evaluation of the likelihood that a nodule is malignant based on its imaging characteristics. This has motivated researchers to develop image analysis research tools, such as nodule detectors and nodule classifiers that can assist radiologists to make accurate assessments of the patient cancer risk. In this work, we propose a two-stage framework that can assess the lung cancer risk associated with a low-dose chest CT scan. At the first stage, our framework employs a nodule detector; while in the second stage, we use both the image area around the nodules and nodule features as inputs to a neural network that estimates the malignancy risk of the whole CT scan. The proposed approach: (a) has better performance than the PanCan Risk Model, a widely accepted method for cancer malignancy assessment, achieving around 7% better Area Under Curve score in two independent datasets we have employed; (b) has comparable performance to radiologists in estimating cancer risk at patient level; (c) employs a novel multi-instance weakly-labeled approach to train the deep learning network that requires confirmed cancer diagnosis only at the patient level (not at the nodule level); and (d) employs a large number of lung CT scans (more than 8000) from heterogeneous data sources (NLST, LHMC, and Kaggle competition data) to validate and compare model performance. AUC scores for our model, evaluated against confirmed cancer diagnosis, range between 82% to 90%.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge