Artur Yakimovich
Single-shot Star-convex Polygon-based Instance Segmentation for Spatially-correlated Biomedical Objects
Apr 16, 2025Abstract:Biomedical images often contain objects known to be spatially correlated or nested due to their inherent properties, leading to semantic relations. Examples include cell nuclei being nested within eukaryotic cells and colonies growing exclusively within their culture dishes. While these semantic relations bear key importance, detection tasks are often formulated independently, requiring multi-shot analysis pipelines. Importantly, spatial correlation could constitute a fundamental prior facilitating learning of more meaningful representations for tasks like instance segmentation. This knowledge has, thus far, not been utilised by the biomedical computer vision community. We argue that the instance segmentation of two or more categories of objects can be achieved in parallel. We achieve this via two architectures HydraStarDist (HSD) and the novel (HSD-WBR) based on the widely-used StarDist (SD), to take advantage of the star-convexity of our target objects. HSD and HSD-WBR are constructed to be capable of incorporating their interactions as constraints into account. HSD implicitly incorporates spatial correlation priors based on object interaction through a joint encoder. HSD-WBR further enforces the prior in a regularisation layer with the penalty we proposed named Within Boundary Regularisation Penalty (WBR). Both architectures achieve nested instance segmentation in a single shot. We demonstrate their competitiveness based on $IoU_R$ and AP and superiority in a new, task-relevant criteria, Joint TP rate (JTPR) compared to their baseline SD and Cellpose. Our approach can be further modified to capture partial-inclusion/-exclusion in multi-object interactions in fluorescent or brightfield microscopy or digital imaging. Finally, our strategy suggests gains by making this learning single-shot and computationally efficient.
Solving the inverse problem of microscopy deconvolution with a residual Beylkin-Coifman-Rokhlin neural network
Jul 03, 2024



Abstract:Optic deconvolution in light microscopy (LM) refers to recovering the object details from images, revealing the ground truth of samples. Traditional explicit methods in LM rely on the point spread function (PSF) during image acquisition. Yet, these approaches often fall short due to inaccurate PSF models and noise artifacts, hampering the overall restoration quality. In this paper, we approached the optic deconvolution as an inverse problem. Motivated by the nonstandard-form compression scheme introduced by Beylkin, Coifman, and Rokhlin (BCR), we proposed an innovative physics-informed neural network Multi-Stage Residual-BCR Net (m-rBCR) to approximate the optic deconvolution. We validated the m-rBCR model on four microscopy datasets - two simulated microscopy datasets from ImageNet and BioSR, real dSTORM microscopy images, and real widefield microscopy images. In contrast to the explicit deconvolution methods (e.g. Richardson-Lucy) and other state-of-the-art NN models (U-Net, DDPM, CARE, DnCNN, ESRGAN, RCAN, Noise2Noise, MPRNet, and MIMO-U-Net), the m-rBCR model demonstrates superior performance to other candidates by PSNR and SSIM in two real microscopy datasets and the simulated BioSR dataset. In the simulated ImageNet dataset, m-rBCR ranks the second-best place (right after MIMO-U-Net). With the backbone from the optical physics, m-rBCR exploits the trainable parameters with better performances (from ~30 times fewer than the benchmark MIMO-U-Net to ~210 times than ESRGAN). This enables m-rBCR to achieve a shorter runtime (from ~3 times faster than MIMO-U-Net to ~300 times faster than DDPM). To summarize, by leveraging physics constraints our model reduced potentially redundant parameters significantly in expertise-oriented NN candidates and achieved high efficiency with superior performance.
Single Exposure Quantitative Phase Imaging with a Conventional Microscope using Diffusion Models
Jun 06, 2024Abstract:Phase imaging is gaining importance due to its applications in fields like biomedical imaging and material characterization. In biomedical applications, it can provide quantitative information missing in label-free microscopy modalities. One of the most prominent methods in phase quantification is the Transport-of-Intensity Equation (TIE). TIE often requires multiple acquisitions at different defocus distances, which is not always feasible in a clinical setting. To address this issue, we propose to use chromatic aberrations to induce the required through-focus images with a single exposure, effectively generating a through-focus stack. Since the defocus distance induced by the aberrations is small, conventional TIE solvers are insufficient to address the resulting artifacts. We propose Zero-Mean Diffusion, a modified version of diffusion models designed for quantitative image prediction, and train it with synthetic data to ensure robust phase retrieval. Our contributions offer an alternative TIE approach that leverages chromatic aberrations, achieving accurate single-exposure phase measurement with white light and thus improving the efficiency of phase imaging. Moreover, we present a new class of diffusion models that are well-suited for quantitative data and have a sound theoretical basis. To validate our approach, we employ a widespread brightfield microscope equipped with a commercially available color camera. We apply our model to clinical microscopy of patients' urine, obtaining accurate phase measurements.
Conditional Variational Diffusion Models
Dec 04, 2023



Abstract:Inverse problems aim to determine parameters from observations, a crucial task in engineering and science. Lately, generative models, especially diffusion models, have gained popularity in this area for their ability to produce realistic solutions and their good mathematical properties. Despite their success, an important drawback of diffusion models is their sensitivity to the choice of variance schedule, which controls the dynamics of the diffusion process. Fine-tuning this schedule for specific applications is crucial but time-costly and does not guarantee an optimal result. We propose a novel approach for learning the schedule as part of the training process. Our method supports probabilistic conditioning on data, provides high-quality solutions, and is flexible, proving able to adapt to different applications with minimum overhead. This approach is tested in two unrelated inverse problems: super-resolution microscopy and quantitative phase imaging, yielding comparable or superior results to previous methods and fine-tuned diffusion models. We conclude that fine-tuning the schedule by experimentation should be avoided because it can be learned during training in a stable way that yields better results.
Phenotype-preserving metric design for high-content image reconstruction by generative inpainting
Aug 01, 2023Abstract:In the past decades, automated high-content microscopy demonstrated its ability to deliver large quantities of image-based data powering the versatility of phenotypic drug screening and systems biology applications. However, as the sizes of image-based datasets grew, it became infeasible for humans to control, avoid and overcome the presence of imaging and sample preparation artefacts in the images. While novel techniques like machine learning and deep learning may address these shortcomings through generative image inpainting, when applied to sensitive research data this may come at the cost of undesired image manipulation. Undesired manipulation may be caused by phenomena such as neural hallucinations, to which some artificial neural networks are prone. To address this, here we evaluate the state-of-the-art inpainting methods for image restoration in a high-content fluorescence microscopy dataset of cultured cells with labelled nuclei. We show that architectures like DeepFill V2 and Edge Connect can faithfully restore microscopy images upon fine-tuning with relatively little data. Our results demonstrate that the area of the region to be restored is of higher importance than shape. Furthermore, to control for the quality of restoration, we propose a novel phenotype-preserving metric design strategy. In this strategy, the size and count of the restored biological phenotypes like cell nuclei are quantified to penalise undesirable manipulation. We argue that the design principles of our approach may also generalise to other applications.
Roadmap on Deep Learning for Microscopy
Mar 07, 2023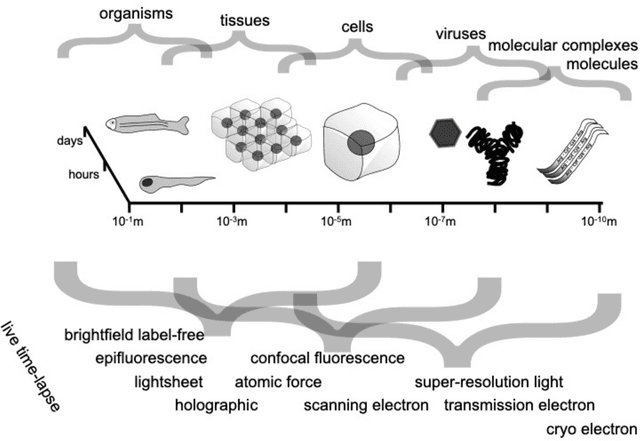
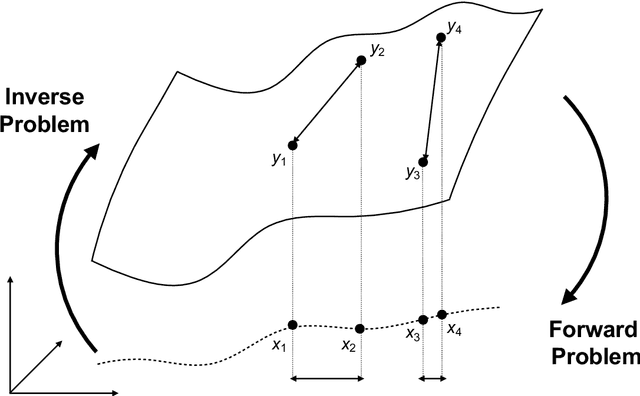
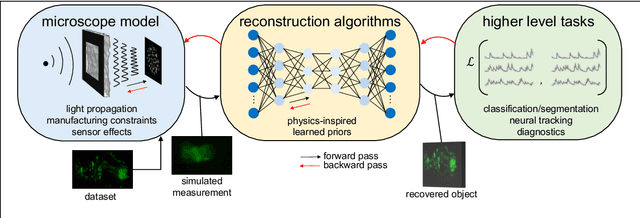
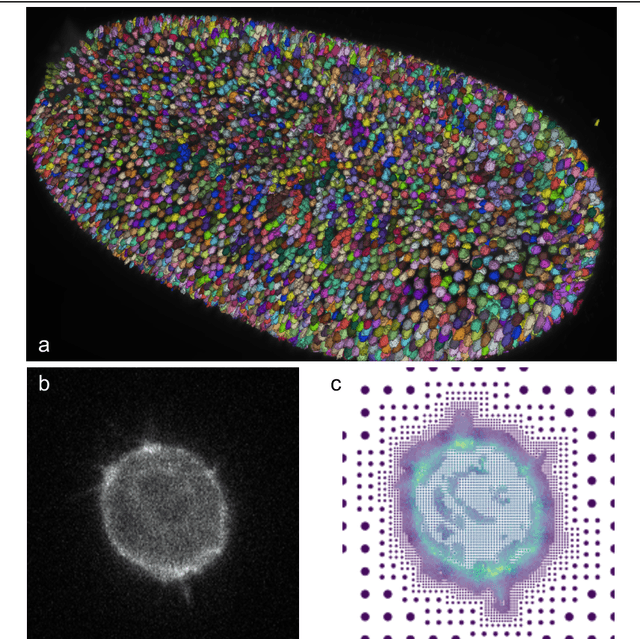
Abstract:Through digital imaging, microscopy has evolved from primarily being a means for visual observation of life at the micro- and nano-scale, to a quantitative tool with ever-increasing resolution and throughput. Artificial intelligence, deep neural networks, and machine learning are all niche terms describing computational methods that have gained a pivotal role in microscopy-based research over the past decade. This Roadmap is written collectively by prominent researchers and encompasses selected aspects of how machine learning is applied to microscopy image data, with the aim of gaining scientific knowledge by improved image quality, automated detection, segmentation, classification and tracking of objects, and efficient merging of information from multiple imaging modalities. We aim to give the reader an overview of the key developments and an understanding of possibilities and limitations of machine learning for microscopy. It will be of interest to a wide cross-disciplinary audience in the physical sciences and life sciences.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge